cnt修饰的ceo2 -ZnO纳米杂化物具有优异的光催化和电催化活性
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
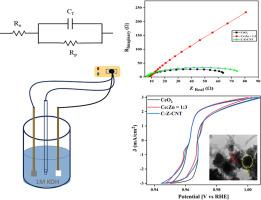
光催化和电化学分析在解决能源和环境挑战方面发挥着重要作用。在这项研究中,我们研究了光催化降解罗丹明B (RhB)染料和CeO₂,CeO₂-ZnO(二元)和CeO₂-ZnO- cnt三元纳米复合材料的电催化性能。虽然在早期的研究中已经报道了CeO₂和CeO₂-ZnO(二元)的合成和基本表征,但本文采用简单的化学方法合成了三元复合材料,并使用x射线衍射(XRD)、拉曼光谱、紫外-可见光谱、透射电子显微镜(TEM)和能量色散光谱(EDS)对其进行了详细的表征。x射线光电子能谱(XPS)研究确定了表面元素组成,并解释了组成元素的氧化态。在日光和紫外光下评估了这三种复合材料的光催化性能,其中三元纳米复合材料在紫外光下对RhB染料的降解效率最高(~ 95%)。利用三乙醇胺(TEA)、异丙醇(IPA)和苯醌(BQ)进行清除率研究,以确定参与光催化过程的关键反应物质。在1 M KOH电解液中,通过析氢反应(HER)和析氧反应(OER)考察了ceo2、ceo2 -ZnO和ceo2 -ZnO- cnt三种样品的电化学性能。该三元复合材料表现出显著的电催化活性,HER的起始电位为0.06 V, OER的起始电位为1.61 V,表明其具有可再生能源转化的潜力。光催化和电化学分析的结合突出了这些纳米复合材料的多功能性,使它们成为能源和环境应用的有希望的候选者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CNT-modified CeO₂-ZnO Nanohybrid for superior photocatalytic and electrocatalytic activity
Photocatalysis and electrochemical analysis play a significant role in addressing energy and environmental challenges. In this study, we investigate the photocatalytic degradation of Rhodamine B (RhB) dye and the electrocatalytic properties of CeO₂, CeO₂-ZnO (binary) and CeO₂-ZnO-CNT ternary nanocomposites. While the synthesis and basic characterization of CeO₂ and CeO₂-ZnO (binary) have already been reported in earlier studies, the ternary composite was synthesized via a simple chemical route and characterized in detail using X-ray diffraction (XRD), Raman spectroscopy, UV–Vis spectroscopy, Transmission Electron Microscopy (TEM) and Energy-Dispersive Spectroscopy (EDS). X-ray photoelectron spectroscopy (XPS) studies were performed to determine the surface elemental composition and explain the oxidation states of the constituent elements. Photocatalytic performance was evaluated for all three composites under sunlight and UV light, with the ternary nanocomposite exhibiting the highest degradation efficiency (∼95 %) under UV light for RhB dye. Scavenger studies using triethanolamine (TEA), isopropanol (IPA), and benzoquinone (BQ) were carried out to determine the key reactive species involved in the photocatalytic process. The electrochemical properties of all three samples-CeO₂, CeO₂-ZnO and CeO₂-ZnO-CNT were examined for the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER) in 1 M KOH electrolyte. The ternary composite exhibited significant electrocatalytic activity with onset potentials of 0.06 V for HER and 1.61 V for OER demonstrating its potential for renewable energy conversion. The combined photocatalytic and electrochemical analysis highlights the versatility of these nanocomposites, making them promising candidates for energy and environmental applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


