偶氮染料在药物制剂中用作赋形剂的电化学行为
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
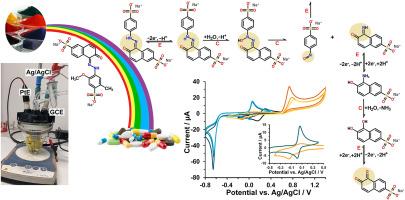
利用循环伏安法,在生理环境缓冲水溶液中的玻碳电极上,对五种偶氮染料的电化学行为进行了全面研究,其中三种为一种(紫红AC、红2G和日落黄FCF),两种为两种偶氮基团(亮黑BN和巧克力棕HT),描述了它们的电化学途径并预测了最可能的反应产物。本研究证实并深化了偶氮染料的电化学性质,揭示了偶氮染料的阴极还原路径为电子传递-电子传递-化学反应(EEC)机制,阳极氧化路径为电子传递-化学反应-化学反应(ECC)机制。因此,对于重氮染料,两种反应途径都通过两步机制进行:EEC-EEC和ECC-ECC。在随后的循环伏安图中观察到典型的氧化还原对,反映了形成的可变取代(磺化)1-氨基-2-萘酚与相应的醌亚胺衍生物之间的准可逆行为。这里令人惊讶的是,除了这些高活性产物外,还可以形成其他有毒的磺化氨基苯(还原)甚至致癌的磺化亚硝基苯(氧化产物)。此外,从单个氧化还原对的循环伏安变化可以推测,上述醌亚胺衍生物很有可能通过水解生成醌类化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrochemical behaviour of azo dyes used as excipients in pharmaceutical preparations
Electrochemical behaviour of five azo dyes, of which three with one (Allura Red AC, Red 2G, and Sunset Yellow FCF) and two with two azo groups (Brilliant Black BN and Chocolate Brown HT), has been exhaustively studied at a glassy carbon electrode in a buffered aqueous solution of physiological environment using cyclic voltammetry to describe their both electrochemical pathways and predict the most probable reaction products. This study has confirmed and deepened recently discovered insights into the electrochemical properties of azo dyes, which indicate an electron-transfer–electron-transfer–chemical reaction (EEC) mechanism in their cathodic reduction pathways and an electron-transfer–chemical reaction–chemical reaction (ECC) mechanism in their anodic oxidation pathways. Therefore, for diazo dyes, both reaction pathways proceed via two-step mechanisms: EEC–EEC and ECC–ECC. Typical redox couples, reflecting quasi-reversible behaviour between formed variable substituted (sulphonated) 1-amino-2-naphthol with the corresponding quinoneimine derivatives, were observed in the subsequent cyclic voltammograms. Here it is striking that in addition to these highly reactive products, other toxic sulphonated aminobenzenes (reduction) or even carcinogenic sulphonated nitrosobenzenes (oxidation products) can also be formed. In addition, it can be assumed with high probability that the formation of quinones can occur by hydrolysis of the above-mentioned quinoneimine derivatives, as evidenced by the shift of cyclic voltammograms for individual redox couples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


