机器学习模型预测肝靶向琼脂糖- cmc - ceo2纳米复合材料的ph响应性药物释放:肝靶向抗氧化打击
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-19
DOI:10.1016/j.jddst.2025.107555
引用次数: 0
摘要
本研究提出了一种创新的羧甲基纤维素/琼脂糖/氧化铈(CMC/Aga/CeO2)纳米载体,用于向肝癌细胞ph敏感递送槲皮素(QC)。该纳米载体表现出出色的载药能力,包封效率达到88.25%,载药量达到51.00%,CeO2的使用显著提高了载药量。理想的物理化学特性包括流体动力直径为193.48 nm,胶体稳定性提高(zeta电位:-61.6 mV)。该系统表现出肿瘤选择性释放动力学,在96小时的持续时间内,在酸性肿瘤pH(5.4)下释放98%的QC,而在生理pH(7.4)下释放64%。体外细胞毒性实验证实了精确靶向,导致HepG2细胞活力降低40%,L929正常细胞活力保留94%。除了这些发现,机器学习建模技术(梯度增强、神经网络、随机森林和支持向量机)被设计用于复制释放动力学,以显著的精度准确表征非线性ph -时间曲线(R2 > 0.97)。这些预测方法为改进现有动力学模型之外的纳米载体设计提供了计算辅助。这个多功能平台有效地缓解了传统QC治疗的一些主要局限性,包括低溶解度、全身毒性和非特异性生物分布,从而为精确肿瘤学提供了一个生物相容性和计算优化的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
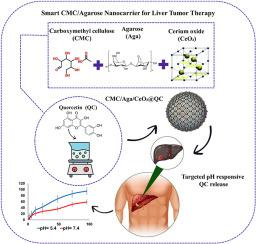
Machine learning models predict pH-responsive drug release from liver-targeted agarose-CMC-CeO2 nanocomposites: Liver-targeted antioxidant strike
This research presents an innovative carboxymethyl cellulose/agarose/cerium oxide (CMC/Aga/CeO2) nanocarrier for the pH-sensitive delivery of quercetin (QC) to hepatocellular carcinoma cells. The nanocarrier exhibited exceptional drug-loading proficiency, achieving 88.25 % entrapment efficiency and 51.00 % loading capacity, which was significantly enhanced by the use of CeO2. The ideal physicochemical characteristics comprised a consistent hydrodynamic diameter of 193.48 nm and elevated colloidal stability (zeta potential: -61.6 mV). The system exhibited tumor-selective release kinetics, discharging 98 % of QC at an acidic tumor pH (5.4) compared to 64 % at physiological pH (7.4) over a 96-h duration. In vitro cytotoxicity experiments validated precise targeting, leading to a 40 % decrease in HepG2 cell viability and a 94 % preservation of L929 normal cell viability. Alongside these findings, machine learning modeling techniques (Gradient Boosting, Neural Networks, Random Forest, and SVM) were devised to replicate release kinetics, accurately characterizing non-linear pH–time profiles with remarkable precision (R2 > 0.97). These prediction methodologies provide computational assistance for enhancing nanocarrier design beyond existing kinetic models. This multi-functional platform effectively mitigates some of the major limitations of conventional QC treatment, including low solubility, systemic toxicity, and non-specific biodistribution, and thereby offers a biocompatible and computationally optimized platform for precision oncology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


