优化酒石酸托特罗定负载sndds提高口服生物利用度:体外和体内研究
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-19
DOI:10.1016/j.jddst.2025.107554
引用次数: 0
摘要
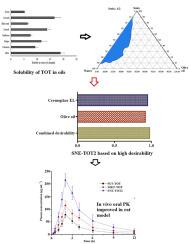
本研究考察了负载酒石酸托特罗定(TOT)的优化SNEDDS的稳定性,促进吸收和高口服生物利用度(BA)。qbd方法优化了鲁棒性组成,并确定了关键因素(橄榄油= X1,焦化剂EL = X2)对集合响应(Y1 =粒径,Y2 = %透光率,Y3 = %捕集效率)的影响。在应力条件下进行了热力学稳定性测试。对优化后的SNE-TOT2进行了配方特性(大小分布、形状和zeta电位)评价,并进行了体外药物释放和体外肠渗透比较研究。体外溶血试验代替急性初步毒性试验。在快速条件下进行了大鼠口服药代动力学和肝毒性研究。热力学稳定的SNE-TOT2(理想性~ 0.97)具有理想的配方属性(小尺寸,159 nm;低多分散性指数,0.235;高zeta电位,- 16.54 mV;高EE, 81%和高透光率)和高效的自乳化性能。X1和X2对Y1-Y3的影响呈二次型,说明需要对这些因素进行优化选择。与SUS-TOT相比,sne - to2表现出异常(非菲克式)药物释放行为(15 min内>; 80%)。sne - to2肠透性最高(219.9±27.21 μg/cm2),吸收优于SUS-TOT(59.2±13.9 μg/cm2)和市售(MKT-TOT)(128.2±23.18 μg/cm2)。此外,与SUS-TOT和MKT-TOT相比,sne - to2改善了药代动力学参数,降低了肝毒性。因此,SNE-TOT2可以作为一种有前景的安全口服产品,改善口服BA。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optimized tolterodine tartrate loaded SNEDDS for enhanced oral bioavailability: In vitro and in vivo studies
The study investigated tolterodine tartrate (TOT)-loaded optimized SNEDDS for improved stability, facilitated absorption, and high oral bioavailability (BA). The QbD-approach optimized the robust composition and identified the impact of critical factors (olive oil = X1 and cremophor EL = X2) on the set responses (Y1 = size, Y2 = %Transmittance, and Y3 = % entrapment efficiency). Thermodynamic stability testing was conducted under stressed conditions. The optimized SNE-TOT2 was evaluated for the formulation characteristics (size distribution, shape, and zeta potential), followed by comparative in vitro drug release and ex vivo intestinal permeation studies. In vitro hemolysis study surrogated acute preliminary toxicity study. Oral pharmacokinetics and hepatotoxicity studies were performed in rats under fast condition. The thermodynamically stable SNE-TOT2 (desirability ∼ 0.97) possessed desired formulations attributes (small size, 159 nm; low polydispersity index, 0.235; high zeta potential, −16.54 mV; high %EE, 81 %, and high %transmittance) and efficient self-emulsification performance. The impact of X1 and X2 on Y1-Y3 was quadratic, indicating the need for optimal selection of these factors. SNE-TOT2 showed anomalous (non-Fickian) drug release behavior (> 80 % within 15 min) and as compared to SUS-TOT. SNE-TOT2 exhibited the highest intestinal permeation (219.9 ± 27.21 μg/cm2), suggesting superior absorption compared with SUS-TOT (59.2 ± 13.9 μg/cm2) and marketed (MKT-TOT) (128.2 ± 23.18 μg/cm2). Moreover, SNE-TOT2 improved pharmacokinetic parameters and reduced hepatic toxicity, compared to SUS-TOT and MKT-TOT. Thus, SNE-TOT2 can be a promising and safe oral product with improved oral BA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


