高效协同策略使高性能富镍单晶lini0.83 co0.11 mn0.060 o2阴极的界面和晶体结构稳定
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
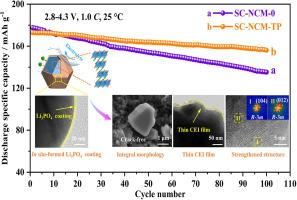
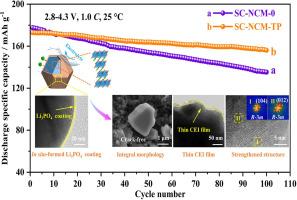
为了解决富镍层状阴极的结构和界面降解问题,设计了Ti4+离子掺杂与原位形成的Li3PO4涂层相结合的协同策略,以调节和优化富镍单晶lini0.83 co0.11 mn0.060 o2 (SC-NCM-TP)阴极。引入的Ti4+离子不仅通过形成更强的Ti-O键增强了晶体结构,抑制了有害的相变,而且通过扩展迁移通道提高了Li+离子的动力学。此外,通过NH4H2PO4处理将表面残余锂盐转化成厚度约为3nm的均匀Li3PO4导电涂层,增强了阴极/电解质界面的稳定性。受益于这些优点,SC-NCM-TP基电池在1.0℃下循环100次后,在25℃下的容量保持率为90.3%,在60℃下的容量保持率为84.6%,在5.0℃下循环200次后,容量保持率仍保持在81.3%。因此,本研究为锂离子电池先进单晶阴极的设计提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effective collaborative strategy enabling stable interphase and crystal structure for high-performance Ni-rich single-crystal LiNi0.83Co0.11Mn0.06O2 cathode
To address the structural and interfacial degradation of Ni-rich layered cathodes, a collaborative strategy combining Ti4+ ions doping with in situ-formed Li3PO4 coating is designed to regulate and optimize Ni-rich single-crystal LiNi0.83Co0.11Mn0.06O2 (SC-NCM-TP) cathode. The introduced Ti4+ ions not only strengthen the crystal framework and inhibit harmful phase transition by forming stronger Ti-O bonds, but also boost Li+ ions kinetics by expanding migration channels. Moreover, a uniform Li3PO4 conductive coating with a thickness of about 3 nm, generated by converting surface residual lithium salts through NH4H2PO4 treatment, enhances cathode/electrolyte interfacial stability. Benefiting from these advantages, the SC-NCM-TP based cell demonstrates exceptional capacity retention ratio of 90.3 % at 25 °C and 84.6 % at 60 °C after 100 cycles under 1.0 C, and maintains excellent capacity retention ratio of 81.3 % after 200 cycles even at 5.0 C. Hence, this work sheds new light on the design of advanced single-crystal cathodes for lithium-ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


