通过支架跳跃疗法发现新型强效茚唑类FXR激动剂
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
farnesoid X受体(FXR)在调节胆汁酸稳态、炎症、纤维化以及葡萄糖和脂质代谢中起着至关重要的作用,将其定位为代谢相关脂肪性肝炎(MASH)治疗的有希望的靶点。LMB763 (niduexor)是诺华公司开发的临床阶段FXR激动剂,在临床前MASH模型中显示出减轻肝脏脂肪变性、炎症和纤维化的疗效。以LMB763为先导化合物,通过支架跳跃策略设计合成了一系列新型化合物。先导化合物E2具有较强的FXR激动剂活性,EC50值为0.097±0.009 μM,具有良好的肝微粒体代谢稳定性。此外,化合物E2对LXRα/β、PPARα/γ/δ、PXR和TGR5等相关核受体具有良好的选择性。体内评价证实,化合物E2改善了高脂肪饮食(HFD)诱导的MASH小鼠模型中的肝脏脂肪变性。这些发现突出了E2作为一个有希望进一步开发的候选者,并为设计用于MASH治疗的选择性FXR激动剂提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
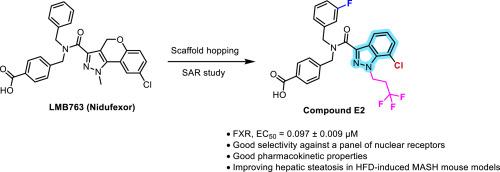
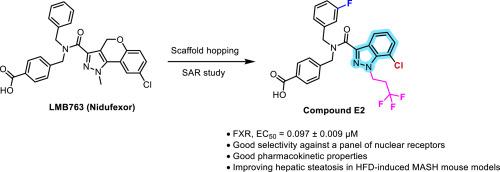
Discovery of novel potent indazole-based FXR agonists via scaffold hopping for MASH treatment
The farnesoid X receptor (FXR) plays a crucial role in regulating bile acid homeostasis, inflammation, fibrosis, as well as glucose and lipid metabolism, positioning it as a promising target for the treatment of Metabolic Associated Steatohepatitis (MASH). LMB763 (Nidufexor), a clinical-stage FXR agonist developed by Novartis, has demonstrated efficacy in alleviating hepatic steatosis, inflammation, and fibrosis in preclinical MASH models. Using LMB763 as a lead compound, a series of novel compounds were designed and synthesized via a scaffold hopping strategy. The lead compound E2 exhibited potent FXR agonist activity with an EC50 value of 0.097 ± 0.009 μM and favorable hepatic microsomal metabolic stability. Additionally, compound E2 displayed good selectivity against related nuclear receptors, including LXRα/β, PPARα/γ/δ, PXR, and TGR5. In vivo evaluation confirmed that compound E2 improved hepatic steatosis in high-fat diet (HFD)-induced MASH mouse model. These findings highlight E2 as a promising candidate for further development and provide valuable insights into the design of selective FXR agonists for MASH treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


