喹啉-5,8-二酮CDC25抑制剂:白血病和患者来源的结肠直肠类器官的有效抗癌药物
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
细胞分裂周期25 (CDC25)磷酸酶是细胞周期蛋白依赖性激酶(CDKs)的关键激活因子和基因组完整性的守护者,使其成为有吸引力的抗癌靶点。在喹啉-5,8-二酮骨架NSC663284 (6a)的基础上,我们合成了具有不同C-6/C-7烷基胺侧链的衍生物。结构活性研究发现,D3a/D3b(2-(4-甲基胡椒苷-1-酰基)乙胺)和D11a/D11b(2-(二甲氨基)乙胺)是最有效的,对CDC25具有低亚微摩尔的抑制作用。在基于细胞的实验中,这些化合物抑制了白血病(IC50为0.21-1.22 μM)和结直肠癌(IC50为0.13-1.50 μM)的活力,同时降低了对正常结肠上皮细胞的毒性。在机制上,这些衍生物阻断cdc25介导的CDK1 Tyr15去磷酸化,延缓G2/M进展,诱导caspase依赖性细胞凋亡伴DNA损伤。细胞毒效力与基线CDC25C表达相关,证实了靶向活性。值得注意的是,在结直肠癌患者来源的类器官中验证了疗效,为患者特异性反应提供了临床相关的见解。总之,这些发现定义了一类新的具有强效和选择性抗癌活性的CDC25抑制剂,推进了下一代白血病和结直肠癌治疗的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
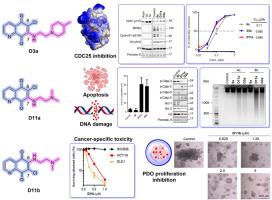
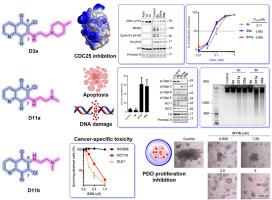
Quinoline-5,8-dione CDC25 inhibitors: Potent anti-cancer agents in leukemia and patient-derived colorectal organoids
Cell division cycle 25 (CDC25) phosphatases are critical activators of cyclin-dependent kinases (CDKs) and guardians of genome integrity, making them attractive anticancer targets. Building on the quinoline-5,8-dione scaffold NSC663284 (6a), we synthesized derivatives with diverse C-6/C-7 alkylamino side chains. Structure–activity studies identified D3a/D3b (2-(4-methylpiperidin-1-yl)ethylamino) and D11a/D11b (2-(dimethylamino)ethylamino) as the most potent, exhibiting low-submicromolar inhibition of CDC25. In cell-based assays, these compounds suppressed leukemia (IC50 0.21–1.22 μM) and colorectal cancer (IC50 0.13–1.50 μM) viability, with reduced toxicity in normal colonic epithelial cells. Mechanistically, these derivatives blocked CDC25-mediated CDK1 Tyr15 dephosphorylation, delayed G2/M progression, and induced caspase-dependent apoptosis with DNA damage. Cytotoxic potency correlated with baseline CDC25C expression, confirming on-target activity. Notably, efficacy was validated in colorectal cancer patient-derived organoids, providing clinically relevant insights into patient-specific responses. Together, these findings define a new class of CDC25 inhibitors with potent and selective anticancer activity, advancing prospects for next-generation therapeutics in leukemia and colorectal cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


