不对称轨道杂化诱导的电子重分配使钠层状氧化物稳定
IF 26
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
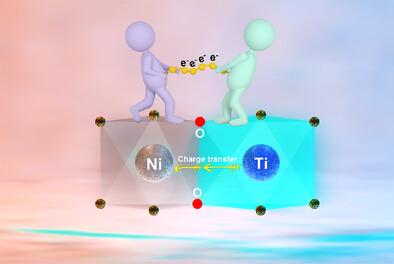
层状过渡金属(TM)氧化物作为钠离子电池(sib)的阴极材料受到了广泛的关注,但复杂的多相转变、严重的结构退化和不稳定的氧氧化还原反应等挑战仍然阻碍了其实际应用。本文提出了一种基于轨道杂化规律的通用电子重分布策略,并考虑到Ni和Ti的不同电负性,引入了NaNi0.5Mn0.35Ti0.15O2 (NNMTO)作为模型阴极。不对称Ni3d-O2p-Ti3d主链(Ni─O─Ti电荷通过桥接氧原子转移)中共价竞争引起的非等效电子分布使Ni和O之间的电子离域,并调节O周围的局部化学环境。同时,通过构建更加刚性的TM─O结构,可以缓解Ni3+O6八面体的协同Jahn-Teller畸变,防止O3′相向有害的O3″相转变。因此,NNMTO表现出持续的可逆能力和显著的循环稳定性,这是基于可逆氧和TM氧化还原过程。本研究从局部化学和轨道杂化调制的角度为构建高性能sib提供了另一种途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Asymmetric-Orbital-Hybridization Induced Electron Redistribution Enabling Stable Sodium Layered Oxides
Layered transition metal (TM) oxides have garnered great attention as viable cathodes for sodium-ion batteries (SIBs), but the challenges of complicated multiphase transitions, severe structural deterioration and unstable oxygen redox reaction still hamper their practical application. Herein, a universal electron redistribution strategy based on the orbital hybridization regulation is proposed and NaNi0.5Mn0.35Ti0.15O2 (NNMTO) is introduced as a model cathode considering the distinct electronegativity between Ni and Ti. The nonequivalent electron distribution induced by the covalency competition within asymmetric Ni3d-O2p-Ti3d backbone (Ni─O─Ti charge transfer via the bridging oxygen atom) delocalizes the electrons between Ni and O and modulates the local chemical environment around O. The enhanced orbital coupling combined with increased Ni─O covalency can not only suppress the over-oxidation of lattice oxygen and improve the reversibility of oxygen redox, but also alleviate the cooperative Jahn–Teller distortion of Ni3+O6 octahedron and prevent the phase transition from O3′ to the detrimental O3″ phase by constructing a more rigid TM─O framework. As a result, NNMTO shows a sustained reversible capacity and remarkable cycling stability that is rooted in reversible oxygen and TM redox processes. This study provides an alternative avenue to construct high-performance SIBs from the perspective of local chemistry and orbital hybridization modulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


