一种Zn(II)基金属有机骨架作为关闭/打开荧光传感器用于选择性检测柠檬酸铁铵和阿司匹林。
IF 4.6
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2025-09-12
DOI:10.1016/j.saa.2025.126914
引用次数: 0
摘要
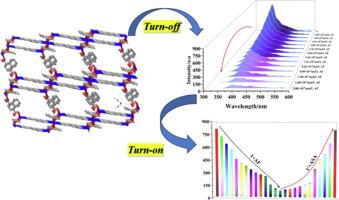
设计具有选择性和敏感性的化学传感器来检测生物分子和苯酚衍生物仍然是一个重大的挑战。本文报道了以1,4-萘二羧酸(H₂L)、1,2-二(吡啶-4-基)乙烷(bpe)和Zn(ClO₄)2·6H₂O为原料,通过水热反应合成了一种高鲁棒性和热稳定性的Zn-金属有机骨架。合成了[Zn₃(L2-)₂(bpe)₂](Zn- mof -1)化合物,并用元素分析、FT-IR、TGA、PXRD、XPS和单晶x射线衍射对其进行了表征。单晶分析表明,Zn-MOF-1具有由C-H···O氢键和π-π堆叠相互作用稳定的三维层状框架结构。值得注意的是,该结构在同一框架内结合了扭曲的八面体和五边形双锥体锌配位几何-这是一个不寻常的特征。拓扑分析证实了(3,6)连接,双重互穿直径(菱形)网络,强调了结构的复杂性和独特性。鉴于Zn-MOF-1优异的发光性能,我们利用多种生物分子进行了传感实验。结果表明,Zn-MOF-1对柠檬酸铁铵(AF)和阿司匹林(ASP)有选择性的分子识别,对AF有“关闭”的发光响应,对ASA有“打开”的发光响应。AF和ASA的检出限分别为4.573 × 10-5 mol/L和4.215 × 10-5 mol/L,对应的Stern-Volmer常数(Ksv)分别为2563.29 M-1和2781.25 M-1。利用实际水样对该传感方法的实际适用性进行了评价。实水样试验回收率为95 ~ 102% %,可靠性较好。时间分辨研究显示AF加入后荧光寿命降低,证实了动态猝灭机制。此外,吸收和发射重叠的分析表明,内部过滤效应(IFE)的贡献。经过IFE校正后,结果显示AF和Zn-MOF-1之间存在真正的分子相互作用。这建立了Zn-MOF-1作为环境和生物分析应用的敏感和选择性荧光传感器。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Zn(II)-based metal–organic framework as a turn-off/on fluorescent sensor for selective detection of ammonium ferric citrate and aspirin
The design of selective and sensitive chemosensor for sensing of biomolecules and phenol derivatives remains a significant challenge. Here, we report on the synthesis of a highly robust and thermally stable Zn-metal organic framework, via a hydrothermal reaction using 1,4-naphthalenedicarboxylic acid (H₂L), 1,2-di(pyridin-4-yl)ethane (bpe), and Zn(ClO₄)₂·6H₂O. The resulting compound [Zn₃(L2−)₂(bpe)₂] (Zn-MOF-1) was synthesized and characterized using elemental analysis, FT-IR, TGA, PXRD, XPS, and single-crystal X-ray diffraction. Single-crystal analysis reveals that Zn-MOF-1 features a three-dimensional (3D) layered framework stabilized by C–H···O hydrogen bonding and π–π stacking interactions. Notably, the structure incorporates both distorted octahedral and pentagonal bipyramidal zinc coordination geometries within the same framework-an uncommon feature. Topological analysis confirms a (3,6)-connected, two-fold interpenetrated dia (diamondoid) network, underscoring the complexity and uniqueness of the structure. Given the excellent luminescent properties of Zn-MOF-1, sensing experiments were conducted using various biomolecules. The results demonstrated selective molecular recognition of Ammonium ferric citrate (AF) and aspirin (ASP) by Zn-MOF-1, exhibiting a ‘turn-off’ luminescence response for AF and a ‘turn-on’ response for ASA. The limits of detection (LOD) were 4.573 × 10−5 mol/L for AF and 4.215 × 10−5 mol/L for ASA, with corresponding Stern–Volmer constants (Ksv) of 2563.29 M−1 and 2781.25 M−1, respectively. The practical applicability of this sensing approach was evaluated using real water samples. Real water sample tests gave recoveries of 95–102 %, confirming reliability. Time-resolved studies revealed a reduced fluorescence lifetime upon AF addition, confirming a dynamic quenching mechanism. Furthermore, analysis of the absorption and emission overlap suggested a contribution from the inner filter effect (IFE). After IFE correction, the results highlighted genuine molecular interactions between AF and Zn-MOF-1. This establishes Zn-MOF-1 as a sensitive and selective fluorescent sensor for environmental and bioanalytical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


