寄主-寄主之间的相互作用在savadiensis和淡水贻贝揭示侵染模式和代谢适应喜马拉雅河流。
IF 2.4
3区 生物学
Q1 ZOOLOGY
引用次数: 0
摘要
本研究调查了Unionicola螨虫与淡水贻贝之间复杂的宿主-寄生虫相互作用,重点研究了来自两条河流的四种贻贝的侵染模式。此外,本研究探讨了寄生在喜马拉雅淡水贻贝上的寄生螨的代谢适应性,重点是产生大分子13-顺式二十二酰胺。corrianus作为主要寄主出现,其侵染动态受环境和形态因素的影响。结合形态学和分子分类,鉴定该螨为缅甸螨(Unionicola (Myanmaratax) savadiensis),并探讨其生化适应性。代谢组学分析强调了鳃相关螨中13-顺式二十二酰胺的存在,表明潜在的宿主特异性适应。这种化合物对螨虫来说是新奇的,表明它与宿主的化学环境有独特的相互作用。贻贝的气相色谱-质谱分析表明存在前体二十二烯酸,但不存在最终化合物,支持螨虫从宿主来源的前体合成13-顺式二十二烯酰胺的假设。在偶然寄主的螨虫中缺乏这种化合物进一步强调了这种代谢适应的特异性。此外,其他分离的化合物被确定为聚合物添加剂,这些化合物以其毒性和内分泌干扰特性而闻名,加剧了对贻贝种群的生态威胁。这项研究强调了寄生的双重生态压力和这些生物的动态,促进了我们对淡水生态系统及其威胁的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
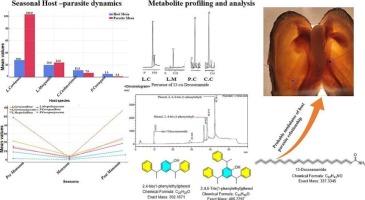
Host-parasite interactions between Unionicola savadiensis and freshwater mussels reveal infestation patterns and metabolic adaptations in Himalayan streams
This study investigates the complex host-parasite interactions between Unionicola mites and freshwater mussels, focusing on infestation patterns across four mussel species from two river streams. Furthermore, this study explores the metabolic adaptations of a parasitic mite infesting Himalayan freshwater mussels, focusing on producing the macromolecule 13-cis-docosenamide. Lamellidens corrianus emerged as the primary host, with environmental and morphometric factors influencing infestation dynamics. Using combined morphological and molecular taxonomy, the mites were identified as Unionicola (Myanmaratax) savadiensis, and their biochemical adaptations were explored. Metabolomics profiling highlighted the presence of 13-cis-docosenamide in gill-associated mites, indicating a potential host-specific adaptation. This compound is novel to mites and suggests a unique interaction with the host’s chemical environment. GC–MS profiling of mussels stated the presence of the precursor docosenoic acid but not the final compound, supporting the hypothesis that mites synthesize 13-cis-docosenamide from host-derived precursors. The absence of this compound in mites from accidental hosts further highlights the specificity of this metabolic adaptation. Additionally, other isolated compounds were identified as polymer additives, which are known for their toxic and endocrine-disrupting properties, exacerbating ecological threats to mussel populations. This study highlights the dual ecological pressures of parasitism and the dynamics of these organisms, advancing our understanding of freshwater ecosystems and their threats.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


