内毒素脂多糖挑战引发斑石斑鱼肠道菌群失调和宿主免疫重塑。
IF 4.3
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2025-09-23
DOI:10.1016/j.cbpc.2025.110364
引用次数: 0
摘要
脂多糖(LPS)是一种细菌内毒素,可以引发水生动物的免疫反应。肠道菌群对鱼类的营养吸收和免疫调节至关重要。本研究以斑石斑鱼(Epinephelus fuscoguttatus)为动物模型,研究LPS对斑石斑鱼肠道菌群组成、功能及头肾基因转录谱的影响。结果表明:LPS刺激使肠道微生物多样性指数(ACE、Chao1、Simpson)略有升高,Shannon指数略有降低;它还增加了变形菌门和有害细菌(弧菌,假单胞菌)的丰度,而减少了厚壁菌门,放线菌门和产生短链脂肪酸的有益细菌(Collinsella, Romboutsia)的丰度。预测肠道菌群的碳水化合物和蛋白质消化吸收功能显著减弱。此外,LPS诱导头肾757个上调基因和379个下调基因,主要涉及DNA复制、氨基酰基- trna生物合成和双组分系统。模式识别受体TLR1/2等免疫相关基因上调,TLR3下调;免疫调节(PIgR、MHC2)、凋亡(Cytc、Casp3、BIRC5)、应激反应(HSP60/70/90、GRP94、Bip、HURP1)和铁稳态(Mfn1、TFR1、Hepc)相关基因上调;补体(C1QL3、C1QBP、C7)、趋化因子及其受体(LECT2、CXCR3/1/4、CCR2)相关基因表达紊乱。Collinsella、Romboutsia、Phyllobacterium、Prevotella 9和Prevotellaceae NK3B31等细菌属群与宿主免疫功能的变化呈正相关。这些结果表明,LPS刺激引起了石斑鱼肠道微生物群的结构扰动,并破坏了石斑鱼头肾的免疫稳态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
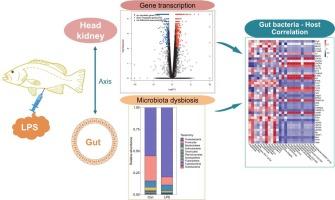
Endotoxin lipopolysaccharide challenge triggers gut microbiota dysbiosis and host immune remodeling in the tiger grouper Epinephelus fuscoguttatus
Lipopolysaccharide (LPS) is a bacterial endotoxin that can trigger immune responses in aquatic animals. The gut microbiota is crucial for nutrient absorption and immune regulation in fish. In this study, the tiger grouper Epinephelus fuscoguttatus was selected as an animal model to investigate the effects of LPS challenge on the composition and function of gut microbiota and the gene transcriptional profiles of head kidney. The results showed that LPS challenge slightly increased gut microbial diversity indices (ACE, Simpson) and decreased Chao1 and Shannon indices; it also increased the abundances of Proteobacteria and harmful bacteria (Vibrio, Pseudomonas), while decreased those of Firmicutes, Actinobacteria and beneficial bacteria (Collinsella, Romboutsia) that produce short-chain fatty acids. The predicted carbohydrate and protein digestion/absorption functions of gut microbiota were significantly weakened. Additionally, LPS challenge induced 757 up- and 379 down-regulated genes in the head kidney, mainly involved in DNA replication, aminoacyl-tRNA biosynthesis and two-component system. Immune-related genes including pattern recognition receptors TLR1/2 was up-regulated but TLR3 was down-regulated; immune regulation (PIgR, MHC2), apoptosis (Cytc, Casp3, BIRC5), stress response (HSP60/70/90, GRP94, Bip, HURP1) and iron homeostasis (Mfn1, TFR1, Hepc) related genes were up-regulated; complement (C1QL3, C1QBP, C7), chemokines and their receptors (LECT2, CXCR3/1/4, CCR2) related genes showed disordered expression. Several bacterial genera groups, such as Collinsella, Romboutsia, Phyllobacterium, Prevotella 9, and Prevotellaceae NK3B31, were positively associated with the changes in host immune function. These findings demonstrated that LPS challenge induced the structural perturbations of gut microbiota, and disrupted the immune homeostasis of the head kidney of the grouper.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


