硝基芳烃的光化学硼化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们描述了一种直接的光驱动策略,将硝基芳烃转化为芳基硼酯。这种转化在温和的环境温度下进行,并能容忍各种各样的官能团。该方法为直接从硝基芳烃制备芳基硼酯提供了一种操作简单实用的替代方法,硝基芳烃是一种容易获得且价格低廉的起始材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。

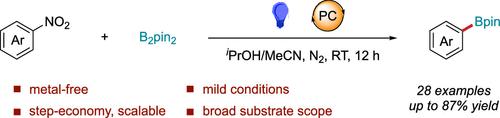
Photochemical Borylation of Nitroarenes
We describe a straightforward light-driven strategy for transforming nitroarenes to aryl boronic esters. This transformation proceeds under mild conditions at ambient temperature and tolerates a wide variety of functional groups. The approach offers an operationally simple and practical alternative for preparing aryl boronic esters directly from nitroarenes, which are readily available and inexpensive starting materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


