表达lmo3的峡周祖细胞维持哺乳动物肠远端细胞生态位的更新和修复
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
位于肠上皮下的肠远端细胞对肠干细胞及其后代发挥着生态位支持作用。它们是沿隐窝绒毛轴划分的异质细胞,但控制这种远端细胞群维持的机制尚不清楚。在这里,我们在发育中的小鼠胚胎中发现了一个独特的上皮下间充质细胞群体,以LIM结构域仅3 (Lmo3)为标志,作为出生后肠远端细胞的细胞起源。Lmo3+细胞在胚胎第13.5天绒毛形成之前就出现了,出生后,它们逐渐获得了肠峡区的空间限制,在那里它们作为长寿命、慢循环的细胞持续存在,供应绒毛周围和隐窝周围的远端细胞。此外,我们发现Lmo3+细胞对组织损伤反应迅速,被激活以促进远端细胞生态位的修复。因此,由Lmo3标记的静止和损伤应答的祖细胞群维持了肠远端细胞的生态位。本文章由计算机程序翻译,如有差异,请以英文原文为准。
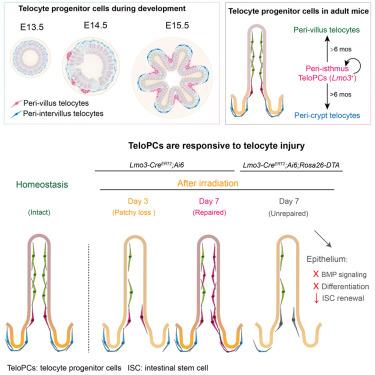
Lmo3-expressing peri-isthmus progenitor cells sustain renewal and repair of the mammalian intestinal telocyte niche
Intestinal telocytes that reside immediately beneath the intestinal epithelium exert niche-supporting roles for intestinal stem cells and their progenies. They are heterogeneous cells compartmentalized along the crypt-villus axis, but the mechanisms governing the maintenance of this telocyte population remain unclear. Here, we identify a distinct population of subepithelial mesenchymal cells in the developing mouse embryo, marked by LIM Domain Only 3 (Lmo3), as the cellular origin of post-natal intestinal telocytes. The Lmo3+ cells emerge prior to villus formation at embryonic day 13.5, and after birth, they progressively acquire a spatial confinement to the intestinal isthmus region, where they persist as long-lived, slow-cycling cells, supplying both peri-villus and peri-crypt telocytes. Further, we show that Lmo3+ cells respond rapidly to tissue damage, becoming activated to promote repair of the telocyte niche. Therefore, a quiescent and damage-responsive progenitor cell population marked by Lmo3 maintains the intestinal telocyte niche.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


