亚砜酰叠氮嘧啶作为致癌转录因子MYC的立体选择性共价不稳定降解剂
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
尽管MYC是一种重要的致癌转录因子驱动癌症,但由于其内在的紊乱和不明确的结构,直接靶向MYC仍然具有挑战性,被认为是“不可药物的”。在蛋白质的非结构化区域内形成的瞬时口袋能否被小分子选择性靶向仍然是一个突出的挑战。在这里,我们建立了一个立体化学配对的螺环氧吲哚偶氮吡啶共价文库,并筛选了该文库用于MYC的降解。我们发现了一种被击中的共价配体KL2-236,它带有一个独特的亚砜酰叠氮化战斗部,作为一种纯粹的MYC/MAX蛋白复合物与MYC结合,在癌细胞中破坏MYC的稳定,抑制MYC的转录活性,并通过靶向内在紊乱的C203和D205残基,以蛋白酶体依赖的方式降解MYC。值得注意的是,这种反应性对于KL2-236与非对映体KL4-019的特定立体异构体最为明显,而后者基本上没有活性。诱变C203和D205完全减弱了kl2 -236介导的MYC降解。我们还优化了KL2-236命中化合物,以产生更有效,选择性和耐用的MYC降解剂KL4-219A。我们的研究结果揭示了MYC中一个新的可配位位点,并表明转录因子中某些内在无序的区域,如MYC,可以被异构独特的手性小分子询问,导致不稳定和降解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
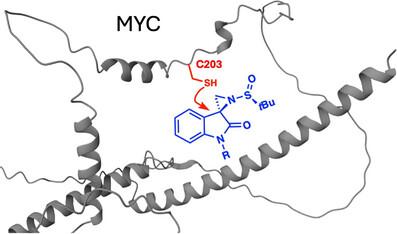
Sulfinyl Aziridines as Stereoselective Covalent Destabilizing Degraders of the Oncogenic Transcription Factor MYC
Although MYC is a significant oncogenic transcription factor driver of cancer, directly targeting MYC has remained challenging due to its intrinsic disorder and poorly defined structure, deeming it “undruggable.” Whether transient pockets formed within unstructured regions of proteins can be selectively targeted with small molecules remains an outstanding challenge. Here, we developed a stereochemically paired spirocyclic oxindole aziridine covalent library and screened this library for degradation of MYC. We identified a hit covalent ligand, KL2-236, bearing a unique sulfinyl aziridine warhead, that engaged MYC as a pure MYC/MAX protein complex, and in cancer cells to destabilize MYC, inhibit MYC transcriptional activity and degrade MYC in a proteasome-dependent manner through targeting intrinsically disordered C203 and D205 residues. Notably, this reactivity was most pronounced for specific stereoisomers of KL2-236 with a diastereomer, KL4-019, that was largely inactive. Mutagenesis of both C203 and D205 completely attenuated KL2-236-mediated MYC degradation. We also optimized our KL2-236 hit compound to generate a more potent, selective, and durable MYC degrader, KL4-219A. Our results reveal a novel ligandable site within MYC and indicate that certain intrinsically disordered regions within transcription factors, such as MYC, can be interrogated by isomerically unique chiral small molecules, leading to destabilization and degradation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


