一种新型Si─B试剂的开发促进了末端炔的反硅化反应
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
1,2-硅烷化是区域和立体选择性生成多功能1-硼基-2-硅基烯烃的有力策略,这是利用硼和硅的正交反应性进行各种下游转化的普遍合成子。然而,它的主要特点是顺式选择性。到目前为止,反选择硅化反应仍然不发达,特别是对于位阻末端炔。本文报道了一种新型Si─B试剂TBSQ在硅上携带一个8-喹啉基导向基团,在pd催化下进行了末端炔的反硅化反应。该策略在广泛的衬底范围内提供独家的e选择性,包括体积较大的炔烃。机理研究表明,这是一条顺式加成/Z→E异构化的复合途径,其中导向基团起着至关重要的作用。由此产生的反式-1-硼基-2-硅基烯烃为选择性下游功能化药学相关分子提供了有价值的构建块。本文章由计算机程序翻译,如有差异,请以英文原文为准。
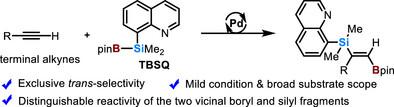
trans-Silaboration of Terminal Alkynes Enabled by Development of a New Si─B Reagent
The 1,2-silaboration of alkynes is a powerful strategy for regio- and stereoselective yielding versatile 1-boryl-2-silyl alkenes, which are ubiquitous synthons leveraging the orthogonal reactivity of boron and silicon for diverse downstream transformations. However, it dominantly features cis-selectivity. trans-Selective silaboration remains underdeveloped to date, especially for sterically hindered terminal alkynes. Herein, we report a Pd-catalyzed trans-silaboration of terminal alkynes using a novel Si─B reagent, TBSQ, bearing an 8-quinolinyl directing group on silicon. This strategy provides exclusive E-selectivity across a broad substrate scope, including bulky alkynes. Mechanistic studies suggest a combined cis-addition/Z→E isomerization pathway, with the directing group playing a crucial role. The resulting trans-1-boryl-2-silyl alkenes serve as valuable building blocks for selective downstream functionalization toward pharmaceutically relevant molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


