局部高浓度电解液中高温锂硫电池失效机理研究
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
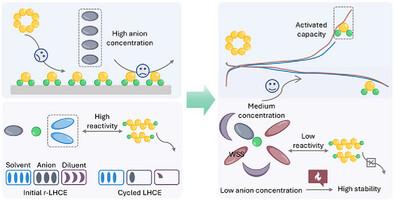
由于较差的热耐久性和加剧的寄生反应,传统的醚基电解质难以在高温下维持锂硫电池的稳定运行。虽然局部高浓度电解质(LHCE)已经成为一种很有前途的提高热稳定性的策略,但它在高温锂硫电池中的应用却收效甚微。本文通过探索硫氧化还原反应和电解质溶剂化化学,揭示了高温锂硫电池在LHCE中的失效机理。在高温下,反应动力学慢和多硫化物反应活性高是导致容量快速劣化的主要因素。为此,研制了阴离子浓度适宜、溶剂化作用弱的二甘醇二丁基醚基局部中浓电解质(B-LMCE)。新型电解质同时实现了快速的阴极动力学和稳定的阳极/电解质界面。在1-3.8 V的电化学电压范围内,Li-S电池在60°C下保持250多个循环的持久循环性能。它们还展示了优越的宽温度操作(0°C - 80°C),同时实现了ah级袋状电池的可行制造。我们的研究为设计实用Li-S电池的极端温度电解质开辟了一条新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Failure Mechanism of High-Temperature Li–S Batteries in Localized High-Concentration Electrolytes
Conventional ether-based electrolytes struggle to sustain steady operation of lithium–sulfur (Li–S) batteries at high temperatures due to inferior thermal durability and aggravated parasitic reactions. Although localized high-concentration electrolyte (LHCE) has emerged as a promising strategy to enhance thermal stability, its deployment in high-temperature (HT) Li–S batteries has met with limited success. Herein, the failure mechanism of HT Li–S batteries in LHCE is revealed via probing sulfur redox reactions and electrolyte solvation chemistry. Slow reaction kinetics and high polysulfide reactivity are determined to be the dominant factors causing the rapid capacity deterioration at high temperatures. To this end, a diethylene glycol dibutyl ether-based localized medium concentration electrolyte (B-LMCE) with suitable anion concentration and weakly solvating effect is developed. The new electrolyte concurrently achieves fast cathode kinetics and stable anode/electrolyte interface. With the assistance of a tailored electrochemical voltage range of 1–3.8 V, Li–S batteries sustain a durable cycling performance over 250 cycles at 60 °C. They also showcase superior wide-temperature operation (0 °C–80 °C) while enabling feasible fabrication of Ah-level pouch cells. Our study opens a new avenue for designing extreme-temperature electrolytes toward pragmatic Li–S batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


