非贵金属te3型金属间化合物(T = Fe, Co; E = Ga, In)的溶液合成和生长研究
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
尽管非贵金属金属间纳米材料具有良好的热电、磁性和催化性能,但由于合成路线具有挑战性,研究很少。为了进一步研究这些材料,我们提出了一种简单、快速、可修改的溶液法合成FeGa3、CoGa3、CoIn3和Fe0.5Co0.5Ga3的方法,并通过pXRD、TEM、SEM、EDS、ICP-MS和XPS对其进行了表征。对这些同结构金属间化合物的反应分析表明,它们依赖于长链仲胺、快速注入速率以及选择性氢化铝还原剂,如二异丁基氢化铝(DIBAL-H),而其他强还原剂,如烷氧基氢化铝和硼氢化铝,则抑制了含ga金属间化合物的形成。我们的研究结果表明,这些反应可以通过共还原或快速汞化(种子生长)机制进行,其中液态Ga纳米种子形成,然后是第一排过渡金属的快速扩散,导致热力学稳定的金属间化合物结晶。这些结果为获取和理解其他未被充分开发的非贵金属金属间纳米材料奠定了基础,我们相信这可能会通过进一步的发展来改善对组成、形态和物理性质的控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
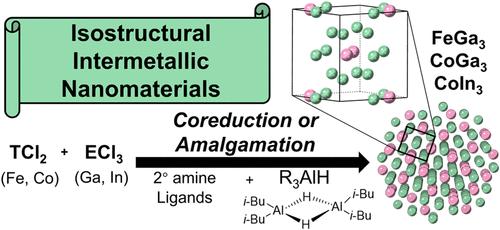
Elucidation of the Solution Synthesis and Growth of Non-Noble Metal TE3-Type Intermetallics (T = Fe, Co; E = Ga, In)
Due to challenging synthetic routes, non-noble metal intermetallic nanomaterials are seldom studied despite having promising thermoelectric, magnetic, and catalytic properties. To initiate these materials’ further study, we present a facile, quick, and modifiable solution-based procedure for the synthesis of FeGa3, CoGa3, CoIn3, and Fe0.5Co0.5Ga3, which are characterized by pXRD, TEM, SEM, EDS, ICP-MS, and XPS. Reaction insights for these isostructural intermetallics demonstrate a reliance on long-chain secondary amines, fast injection rates, as well as select aluminum hydride reductants, such as diisobutylaluminum hydride (DIBAL-H), whereas other strong reductants like alkoxyaluminum hydrides and borohydrides inhibit the formation of Ga-containing intermetallics. Our results suggest that these reactions can be tailored to proceed through either a coreduction or rapid amalgamation (seeded growth) mechanism, in which liquid Ga nanoseeds are formed, followed by rapid diffusion of the first-row transition metals, leading to crystallization of a thermodynamically stable intermetallic. These results lay foundational groundwork for accessing and understanding other underexplored non-noble metal intermetallic nanomaterials, and we believe it may be succeeded by further developments to improve control over composition, morphology, and thus physical properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


