异构体醚溶剂在快速充电和低温SiOx阳极中的空间选择性
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
SiOx阳极有望成为高能量密度电池,但由于SiOx/电解质界面不稳定以及电解质中Li+传输缓慢,因此在快速充电和低温条件下会受到影响。鉴于电解质的重要性,我们提出了一种针对异构体的立体选择溶剂策略,并筛选了优化的溶剂。以具有相同分子式(C5H10O)的四氢吡喃(tetrahydropyran)和2-甲基四氢呋喃(MTHF)两种环醚为研究对象,探讨了溶剂结构与电化学性能之间的复杂关系。MTHF中−CH3引起的位阻效应削弱了溶剂与Li+的配位,有利于形成阴离子衍生的溶剂化结构和具有高机械稳定性的富无机界面相。因此,基于mthf的电解质使LiFePO4|SiOx袋状电池具有快速充电能力(6℃下容量保持70%)和低温性能(- 50℃下放电容量保持46%)。这种同分异构体的立体选择洞察力将分子结构与界面化学和电化学性能联系起来,指导极端条件下高能量密度电池的电解质设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
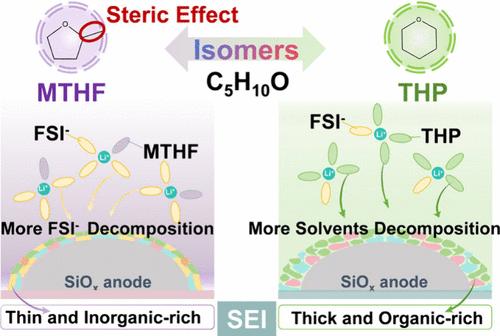
Steric Selectivity of Isomeric Ether Solvents for Fast-Charging and Low-Temperature SiOx Anodes
SiOx anodes promise high-energy-density batteries but suffer under fast-charging and low-temperature conditions because of an unstable SiOx/electrolyte interphase and sluggish Li+ transport in the electrolyte. Given the importance of the electrolyte, we propose an isomer-specific steric-selective solvent strategy and screen the optimized solvent. The intricate relationship between solvent structure and electrochemical performance was explored based on two isomeric cyclic ethers with the same molecular formula (C5H10O): tetrahydropyran and 2-methyltetrahydrofuran (MTHF). The steric hindrance effect induced by −CH3 in MTHF weakens the coordination between solvent and Li+, facilitating the formation of anion-derived solvation structures and a robust inorganic-rich interphase with high mechanical stability. Consequently, the MTHF-based electrolyte enables the LiFePO4|SiOx pouch cell with fast-charging capability (70%-capacity retention at 6 C) and low-temperature performance (46%-discharge-capacity retention under −50 °C). This isomeric steric-selective insight links molecular structure to interfacial chemistry and electrochemical performance, guiding electrolyte design for high-energy-density batteries under extreme conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


