水稻PHT1吸收磷素的低温电镜结构和动态基础
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
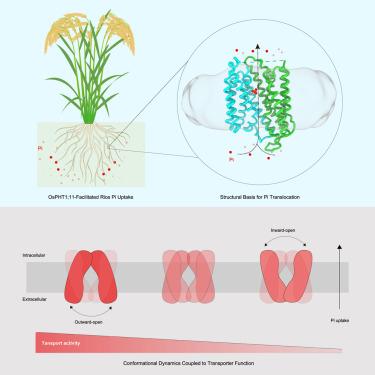
磷是植物必需的大量营养元素,主要通过根系转运体以无机磷酸盐的形式从土壤中吸收。尽管对这些转运体作为开发高效Pi作物的目标进行了数十年的研究,但它们进口Pi的机制仍然知之甚少。在这里,我们展示了水稻Pi进口商OsPHT1的低温电镜(cryo-EM)结构;11在pi束缚和非束缚形式下,表征其构象动力学,并证明这些动力学如何促进其传递功能。Pi是通过在植物中发现的保守残基来识别的,它的易位是通过典型的交替通路机制来促进的。单分子荧光共振能量转移(smFRET)分析表明,这种转运体经历了动态构象波动,这与它的Pi转运能力有不同的联系,细胞外开放构象的优势有利于Pi转运,而更多的细胞内开放构象阻碍了Pi转运。这些发现突出了运输活性的关键构象决定因素,并为植物的Pi摄取提供了机制见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cryo-EM structure and dynamic basis of phosphate uptake by PHT1 in rice
Phosphorus is an essential macronutrient for plants, primarily absorbed from the soil as inorganic phosphate (Pi) through root-located Pi transporters. Despite decades of research into these transporters as targets for developing Pi-efficient crops, their mechanisms for Pi import remain poorly understood. Here, we present the cryo-electron microscopy (cryo-EM) structures of the rice Pi importer OsPHT1;11 in both Pi-bound and unbound forms, characterize its conformational dynamics, and demonstrate how these dynamics contribute to its transport function. Pi is recognized through conserved residues found in plants, with its translocation facilitated by a typical alternating-access mechanism. Single-molecule fluorescence resonance energy transfer (smFRET) analyses show that this transporter undergoes dynamic conformational fluctuations, which are differentially linked to its Pi transport capability, with a predominance of extracellular open conformations favoring Pi transport, while more populated intracellular open conformations hinder it. These findings highlight key conformational determinants of transport activity and provide mechanistic insights into Pi uptake in plants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


