脆弱拟杆菌蛋白酶激活宿主PAR2诱导肠道疼痛和炎症
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
蛋白酶激活受体2 (PAR2)是肠屏障功能、炎症和疼痛的中枢调节因子。肠道蛋白水解和PAR2信号的上调与炎症性肠病(IBDs)和肠易激综合征(IBS)有关,这些疾病通常与肠道微生物组改变有关。为了确定PAR2活性的潜在细菌调节因子,我们开发了一种PAR2加工的功能测定方法来筛选不同的肠道微生物库。我们鉴定出多种细菌分泌能够切割宿主PAR2的蛋白酶。利用一种共价不可逆抑制剂的化学蛋白质组学分析,我们发现了一种以前未被表征的脆弱拟杆菌丝氨酸蛋白酶1 (Bfp1),并表明它在多细胞和小鼠模型中切割和激活PAR2。Bfp1裂解PAR2破坏肠道屏障,使痛觉感受器敏感,引发结肠炎症和腹痛。总的来说,我们的研究结果揭示了bfp1介导的PAR2加工作为肠道宿主-共生相互作用的一个轴,有可能成为IBD或IBS治疗干预的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
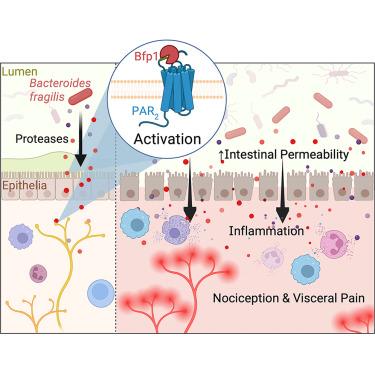
A Bacteroides fragilis protease activates host PAR2 to induce intestinal pain and inflammation
Protease-activated receptor 2 (PAR2) is a central regulator of intestinal barrier function, inflammation, and pain. Upregulated intestinal proteolysis and PAR2 signaling are implicated in inflammatory bowel diseases (IBDs) and irritable bowel syndrome (IBS), conditions often associated with gut microbiome alterations. To identify potential bacterial regulators of PAR2 activity, we developed a functional assay for PAR2 processing to screen a library of diverse gut microbes. We identify multiple bacteria that secrete proteases capable of cleaving host PAR2. Using chemoproteomic profiling with a covalent irreversible inhibitor, we uncovered a previously uncharacterized Bacteroides fragilis serine protease 1 (Bfp1) and show that it cleaves and activates PAR2 in multicellular and murine models. PAR2 cleavage by Bfp1 disrupts the intestinal barrier, sensitizes nociceptors, and triggers colonic inflammation and abdominal pain. Collectively, our findings uncover Bfp1-mediated PAR2 processing as an axis of host-commensal interaction in the gut that has the potential to be targeted for therapeutic intervention in IBD or IBS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


