一种工程单波长可激发双色荧光探针,用于同时成像铁下垂过程中的极性和粘度动力学
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
了解像铁下垂这样复杂的生物过程需要同时监测微环境参数,特别是极性和粘度。然而,现有的荧光探针缺乏独立、实时跟踪的多功能。为了解决这个问题,我们开发了CouPy+,这是第一个单分子探针,可以对铁下垂过程中的极性和粘度动力学进行双色成像。结合扭曲分子内电荷转移(TICT)机制和分子转子,CouPy+在单一激发下发出明显的绿色(极性敏感)和深红色(粘度敏感)信号。该设计允许在活细胞中并发但独立的参数可视化,克服了传统工具的局限性。CouPy+在一个激发下表现出双发射(绿色表示极性,深红色表示粘度),能够在活细胞中同时独立跟踪。线粒体定位,它通过发射差异区分癌细胞和正常细胞。在铁下垂过程中,CouPy+首次实现了实时双色可视化,显示核极性降低和黏度增加。体内研究通过检测斑马鱼中铁中毒相关变化证实了其效用。对照实验验证了探针的特异性,极性和粘度响应之间没有交叉干扰。在活细胞中,在铁下垂期间,极性依赖的绿色发射降低到基线水平的25%,而粘度依赖的红色发射增加了15%。延时成像捕捉到动态参数变化,突出了CouPy+对微环境变化的敏感性。此外,探针的光稳定性和低细胞毒性确保了可靠的长期成像。它的双响应能力为铁下垂过程中极性和粘度的时空耦合提供了前所未有的见解,这是以前单参数探针无法实现的壮举。CouPy+是第一个用于铁下垂过程中极性和粘度双色成像的单分子探针,解决了多功能工具的关键空白。它的tict转子设计为未来的多参数探头建立了蓝图。CouPy+提供了一个强大的工具,可以同时跟踪活细胞和斑马鱼的多个微环境参数,促进对铁下垂和相关病理的更深入的机制理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

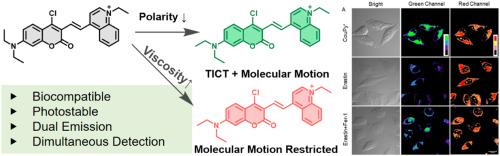
An engineered single-wavelength-excitable dual-color fluorescent probe for simultaneous imaging of polarity and viscosity dynamics during ferroptosis
Understanding complex biological processes like ferroptosis requires simultaneous monitoring of microenvironmental parameters, particularly polarity and viscosity. However, existing fluorescent probes lack multifunctionality for independent, real-time tracking. To address this, we developed CouPy+, the first single-molecule probe enabling dual-color imaging of polarity and viscosity dynamics during ferroptosis. Combining a twisted intramolecular charge transfer (TICT) mechanism with molecular rotors, CouPy + emits distinct green (polarity-sensitive) and deep-red (viscosity-sensitive) signals under a single excitation. This design allows concurrent yet independent parameter visualization in live cells, overcoming limitations of traditional tools. CouPy + exhibits dual-emission (green for polarity, deep-red for viscosity) under one excitation, enabling simultaneous, independent tracking in live cells. Mitochondria-localized, it discriminated cancer cells from normal cells via emission differences. During ferroptosis, CouPy + achieved the first real-time, two-color visualization, revealing concurrent nuclear polarity decreases and viscosity increases. In vivo studies confirmed its utility by detecting ferroptosis-associated changes in zebrafish. Control experiments validated the probe's specificity, with no cross-interference between polarity and viscosity responses. In living cells, polarity-dependent green emission decreased to 25 % of baseline levels, while viscosity-dependent red emission increased by 15 % during ferroptosis. Time-lapse imaging captured dynamic parameter shifts, highlighting CouPy+’s sensitivity to microenvironmental changes. Additionally, the probe's photostability and low cytotoxicity ensured reliable long-term imaging. Its dual-response capability provides unprecedented insights into the spatiotemporal coupling of polarity and viscosity during ferroptosis, a previously unattainable feat with single-parameter probes. CouPy+ is the first single-molecule probe for dual-color imaging of polarity and viscosity during ferroptosis, addressing a critical gap in multifunctional tools. Its TICT-rotor design establishes a blueprint for future multi-parameter probes. CouPy + provides a powerful tool for simultaneously tracking multiple microenvironmental parameters in live cells and zebrafish, facilitating a deeper mechanistic understanding of ferroptosis and related pathologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


