黄素光催化酚类化合物的苯基功能化、螺旋环化和螺旋环氧化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
对醌类化合物(p-QMs)是有机合成中用途广泛的瞬态亲电脱芳中间体,可实现一系列转化,如环加成、亲核加成和级联反应。通常,这些短暂的物种是通过酸或碱介导的活化,在苯基位置上用离去基(如羟基、卤化物或磺酸)预官能团化的苯酚,或通过使用有毒重金属盐(如铅、银或铋)氧化苯酚,在原位产生的。我们报道了一种直接从简单的酚前体原位生成p-QMs的光催化方法,从而避免了对苯基预功能化或过渡金属试剂的需要。光生成的p-QMs进行了有效的转化,包括苯基功能化、螺旋环化和螺旋环氧化,从而快速获得苯基支架和结构复杂的富含sp3的螺旋环框架。药物和天然产物的后期功能化进一步证明了这种方法的合成效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
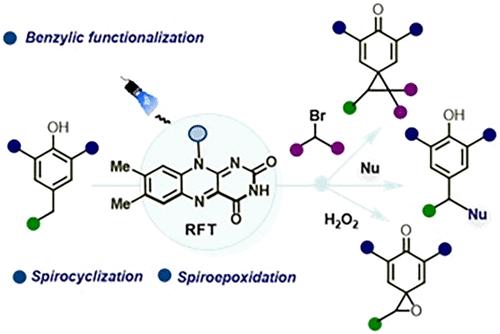
Flavin-Photocatalyzed Benzylic Functionalization, Spirocyclization, and Spiroepoxidation of Phenols
para-Quinone methides (p-QMs) are versatile, transient electrophilic dearomatized intermediates in organic synthesis, enabling a range of transformations, such as cycloadditions, nucleophilic additions, and cascade reactions. Conventionally, these ephemeral species are generated in situ via acid- or base-mediated activation of phenols prefunctionalized with a leaving group (e.g., hydroxyl, halide, or sulfonate) at the benzylic position or through oxidation of phenols employing toxic heavy metal salts (e.g., lead, silver, or bismuth). We report a photocatalytic approach for in situ generation of p-QMs directly from simple phenolic precursors, thereby circumventing the need for benzylic prefunctionalization or transition-metal reagents. The photogenerated p-QMs undergo efficient transformations, including benzylic functionalization, spirocyclization, and spiroepoxidation, delivering rapid access to benzylic scaffolds and structurally complex sp3-rich spirocyclic frameworks. The synthetic utility of this approach is further demonstrated by the late-stage functionalization of drugs and natural products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


