不对称转移加氢合成抗β-羟基色氨酸的动力学解析。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
描述了一种通过ru催化的不对称转移氢化-动态动力学分解(ATH-DKR)级联反应,通过相应的α-氨基-β-酮酯获得抗β-羟基色氨酸的一般方法。必需的起始材料是通过一个强大的,可扩展的,不需要色谱的三步方法从吲哚-3-羧酸制备的。对ATH-DKR反应进行了全面的HTE筛选/优化,以保护基团和取代模式为依据,制备了一系列收率很高、对映选择性和非对映选择性高的类似物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
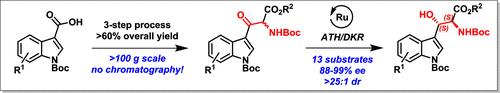
Synthesis of Anti-β-Hydroxytryptophans by Asymmetric Transfer Hydrogenation via Dynamic Kinetic Resolution
A general approach to anti-β-hydroxytryptophans via the corresponding α-amino-β-keto esters through a Ru-catalyzed asymmetric transfer hydrogenation-dynamic kinetic resolution (ATH-DKR) cascade is described. The requisite starting materials are prepared through a robust, scalable, and chromatography-free three-step approach from indole-3-carboxylic acids. A thorough HTE screening/optimization of the ATH-DKR reaction, with regard to protecting groups and substitution patterns, was undertaken to prepare an array of analogs in very good yields and with high enantio- and diastereoselectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


