超越Michaelis-Menten:改进的酶动力学方程改善了自下而上PBPK模型中药物代谢预测。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
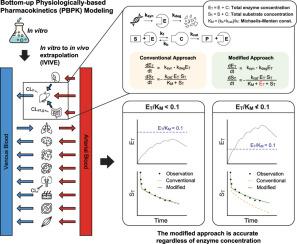
基于生理的药代动力学(PBPK)模型是一种广泛使用的方法,它动态地整合各种生物参数来预测药物及其代谢物的药代动力学特征。传统上,PBPK模型依赖于Michaelis-Menten (MM)方程来描述酶的速率过程,如运输、代谢和分泌。然而,MM方程假设酶浓度(ET)大大低于MM常数(KM)。在体内经常违反这一条件,导致预测清除率和药物-药物相互作用不准确。为了解决这些不准确性,目前的PBPK方法使用I期临床试验数据进行参数优化。然而,这与自下而上的从临床前数据预测人体药代动力学在人体试验之前的范式冲突。在这里,我们通过在PBPK建模框架内实现一个修改的代谢率方程来解决这一冲突。与MM方程不同,修正后的方程即使在ET与KM相当的情况下仍然有效,从而提高了静态模型的预测精度。我们的研究结果表明,即使在动态PBPK建模中,特别是在MM方程假设无效的情况下,使用改进的方程也优于传统的基于MM的方法。基于这些发现,我们提出了扩展指南,以扩大使用修正方程的PBPK建模的适用性。这一进展为药代动力学研究做出了重大贡献,并增强了PBPK模型在药物开发中的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Beyond Michaelis-Menten: A modified enzyme kinetics equation improves drug metabolism prediction in bottom-Up PBPK modeling
Physiologically based pharmacokinetic (PBPK) models are widely used methodology that dynamically integrates diverse biological parameters to predict pharmacokinetic profiles of drugs and their metabolites. Traditionally, PBPK models rely on the Michaelis-Menten (MM) equation to describe enzymatic rate processes such as transport, metabolism, and secretion in terms of intrinsic clearance. However, the MM equation assumes that enzyme concentrations () are substantially lower than the MM constant (). This condition is often violated in vivo, resulting in inaccuracies in predicting clearance and drug-drug interactions. To address these inaccuracies, current PBPK approaches employ parameter optimization using phase I clinical trial data. However, this conflicts with the bottom-up paradigm of predicting human pharmacokinetics from preclinical data prior to human trials. Here, we resolve this conflict by implementing a modified metabolic rate equation within the PBPK modeling framework. Unlike the MM equation, the modified equation remains valid even when is comparable to , thereby improving prediction accuracy in static models. Our results demonstrate that using the modified equation outperforms the conventional MM-based method even in dynamic PBPK modeling, particularly in scenarios where the MM equation's assumptions are invalid. Based on these findings, we propose expanded guidelines to broaden the applicability of PBPK modeling using the modified equation. This advancement offers a significant contribution to pharmacokinetic research and enhances the utility of PBPK models in drug development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


