亚致死杀虫剂胁迫改变了害虫桃蚜(Myzus persicae)的灭绝率,但没有改变其基因突变率。
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
害虫通过选择作用于突变产生的遗传变异,进化出对杀虫剂的遗传抗性。据推测,暴露于低剂量农药可能会增加害虫基因组的突变率并加速抗性进化。然而,农药胁迫对昆虫突变率的影响尚未得到实证检验。在这里,我们利用新的、高质量的蚜虫基因组资源,结合长期的突变积累实验,来调查暴露于杀虫剂和未暴露于杀虫剂的蚜虫系的自发遗传和表观遗传突变率。我们的数据显示,多代接触亚致死浓度的新烟碱类杀虫剂吡虫啉不会增加桃蚜的基因突变率。相反,我们表明,吡虫啉暴露导致显着较低的增殖率。这些发现揭示了害虫中(epi)突变率的起源和组成,并挑战了农药暴露与突变率之间的联系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
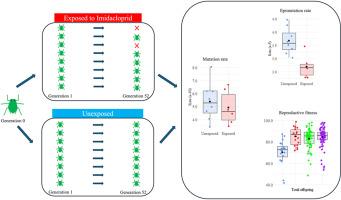
Sub-lethal insecticide stress alters epimutation rate but not genetic mutation rate in the pest insect Myzus persicae
Insect pests evolve heritable resistance to insecticides through selection acting on genetic variation generated by mutation. It has been hypothesised that exposure to low doses of pesticides may increase mutation rate in pest genomes and accelerate resistance evolution. However, the impact of pesticide-induced stress on the mutation rate of insects has never been empirically tested. Here we leverage new, high-quality genomic resources for the aphid pest Myzus persicae in conjunction with long-term mutation accumulation experiments to interrogate spontaneous genetic and epigenetic mutation rates in insecticide-exposed and unexposed aphid lines. Our data reveal that multigenerational exposure of Myzus persicae to sublethal concentrations of the neonicotinoid insecticide imidacloprid does not increase genetic mutation rate. Rather we show that imidacloprid exposure results in a significantly lower epimutation rate. These findings reveal the rate of origin and composition of (epi)mutations arising in a pest insect and challenge the proposed link between pesticide exposure and the rate of mutation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


