Ras 1激酶抑制因子(KSR1)通过激活Ras /RAF/MEK/ERK信号通路促进肝癌的发生
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
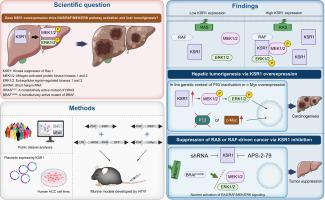
背景和目的在各种人类癌症中经常观察到RAS和RAF基因的突变,导致RAS/RAF/MEK/ERK信号通路的持续激活并驱动肿瘤发生。有趣的是,尽管RAS/RAF/MEK/ERK信号频繁激活,肝细胞癌(HCC)仍显示RAS和RAF的罕见突变。在这里,我们发现Ras 1的激酶抑制因子(KSR1)在肝脏通路激活和肿瘤发生中起关键作用。方法利用公开的数据库分析人类癌症的基因表达数据。建立稳定表达KSR1的HCC细胞系,评估RAS/RAF/MEK/ERK信号活性。采用水动力尾静脉注射法诱导C57BL/6雄性小鼠肝癌。荷瘤肝脏用福尔马林固定,进行H&;E染色和免疫组织化学处理。结果sksr1在人HCC中频繁表达上调(n = 366, p <0.001),与RAS/RAF/MEK/ERK信号通路的激活密切相关(n = 50, p <0.001)。KSR1的异位表达导致HCC细胞和小鼠肝脏中MEK1/2和ERK1/2磷酸化增加,证实了信号通路的激活。小鼠肝脏中KSR1过表达、P53失活或c-Myc过表达导致HCC的发生,HCC表现为RAS/RAF/MEK/ERK信号激活(每组10只小鼠)。值得注意的是,KSR1过表达诱导的MEK磷酸化和肝脏肿瘤发生程度与激活的RAF (MEK1/2的真正激酶)相同。有趣的是,激活的RAS或RAF诱导的肝脏肿瘤可以通过KSR1敲低或KSR1的药理抑制有效地抑制(n = 10, p <0.01),这突出了KSR1作为途径激活驱动的癌症的治疗靶点的潜力。结论KSR1过表达激活RAS/RAF/MEK/ERK信号通路,在RAS或RAF不突变的情况下驱动肝脏肿瘤发生。研究结果表明,Ras 1激酶抑制因子(KSR1)在肝脏中是一种致癌驱动因子,也是Ras /RAF/MEK/ERK信号通路的关键激活因子。这项研究增强了我们对支架蛋白在促进致癌信号级联反应中所起作用的理解。成功使用KSR的药理学抑制剂来抑制由RAS或RAF驱动的体内肿瘤生长,突出了KSR1作为可药物靶点的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinase suppressor of Ras 1 (KSR1) promotes liver carcinogenesis via activation of the RAS/RAF/MEK/ERK signaling pathway
Background & Aims
Mutations in the RAS and RAF genes are frequently observed in various human cancers, leading to persistent activation of the RAS/RAF/MEK/ERK signaling pathway and driving tumorigenesis. Intriguingly, hepatocellular carcinoma (HCC) reveals rare mutations in RAS and RAF, despite frequent activation of the RAS/RAF/MEK/ERK signaling. Here, we identify kinase suppressor of Ras 1 (KSR1) as a pivotal player in pathway activation and tumorigenesis in the liver.
Methods
Gene expression data from human cancers were analyzed using publicly available databases. HCC cell lines stably expressing KSR1 were established to evaluate RAS/RAF/MEK/ERK signaling activity. Liver cancer was induced in C57BL/6 male mice via hydrodynamic tail vein injection. Tumor-bearing livers were formalin-fixed and processed for H&E staining and immunohistochemistry.
Results
KSR1 expression was frequently upregulated in human HCC (n = 366, p <0.001) and strongly associated with activation of the RAS/RAF/MEK/ERK signaling pathway (n = 50, p <0.001). Ectopic expression of KSR1 led to increased phosphorylation of MEK1/2 and ERK1/2 in HCC cells and murine livers, confirming activation of the signaling pathway. Overexpression of KSR1 in murine livers in conjunction with P53 inactivation or c-Myc overexpression led to the development of HCC, which exhibited activated RAS/RAF/MEK/ERK signaling (n = 10 mice per group). Notably, the degrees of MEK phosphorylation and tumorigenesis in the liver induced by KSR1 overexpression were equivalent to those by an activated RAF, the bona fide kinase of MEK1/2. Intriguingly, liver tumors induced by activated RAS or RAF were efficiently suppressed by KSR1 knockdown or pharmacological inhibition of KSR1 (n = 10, p <0.01), highlighting the potential of KSR1 as a therapeutic target for cancers driven by pathway activation.
Conclusions
Overexpression of KSR1 activates the RAS/RAF/MEK/ERK signaling pathway and drives hepatic tumorigenesis in the absence of mutations in RAS or RAF.
Impact and implications
Our findings identify kinase suppressor of Ras 1 (KSR1) as an oncogenic driver in the liver and a key activator of the RAS/RAF/MEK/ERK signaling pathway. This study enhances our understanding of the role that scaffold proteins play in promoting oncogenic signaling cascades. The successful use of a pharmacological inhibitor of KSR to suppress in vivo tumor growth driven by RAS or RAF highlights the potential of KSR1 as a druggable target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


