电化学传感中的选择性分子相互作用:dna模板化Hg(II)捕获和酶驱动的SeO42-还原去除汞
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
了解和控制生物电子界面上的选择性分子相互作用对于开发高性能电化学传感器至关重要。在这项工作中,我们提出了一种dna酶功能化的金电极,用于汞(Hg(II))的双模式检测和沉淀。该传感器利用富含胸腺嘧啶的DNA链选择性捕获Hg(II),形成稳定的T-Hg(II)- t复合物,同时固定化酶催化硒酸盐(SeO42-)选择性还原为亚硒酸盐(SeO32-)。原位生成的SeO32-与结合的Hg(II)发生反应,触发HgSeO3的沉淀,促进汞从DNA支架上的可控释放。电化学阻抗谱(EIS)和循环伏安法(CV)显示了不同的电荷转移电阻(RCT)变化,对应于Hg(II)结合、SeO42-还原和HgSeO3沉淀。该传感器具有优异的选择性,在亚纳摩尔范围内的检测极限,以及来自竞争金属离子和氧离子的强抗干扰性能。环境样品测试进一步验证了其适用性,回收率高(90.3 ~ 109.8%)。结果表明,ICP-MS测量结果与常规Hg(II)检测方法非常吻合。这项工作通过整合分子识别和内在解毒机制来推进dna -酶介导的电化学传感。这些发现为下一代生物电子平台提供了有希望的基础,使无机汞和硒酸盐的实时监测成为可能,并提供了一种潜在的除汞方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。

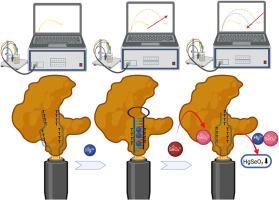
Selective molecular interplay in electrochemical sensing: DNA-templated Hg(II) capture and enzyme-driven SeO42− reduction for mercury removal
Understanding and controlling selective molecular interactions at bioelectronic interfaces are crucial for developing high-performance electrochemical sensors. In this work, we present a DNA-enzyme functionalized gold electrode for the dual-mode detection and precipitation of mercury (Hg(II)). The sensor utilizes thymine-rich DNA strands to selectively capture Hg(II), forming stable T-Hg(II)-T complexes, while an immobilized enzyme catalyzes the selective reduction of selenate (SeO42−) to selenite (SeO32−). The in situ-generated SeO32− reacts with the bound Hg(II), triggering the precipitation of HgSeO3 and facilitating the controlled release of mercury from the DNA scaffold. Electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) revealed distinct charge transfer resistance (RCT) variations, corresponding to Hg(II) binding, SeO42− reduction, and HgSeO3 precipitation. The sensor exhibited exceptional selectivity, a detection limit in the sub-nanomolar range, and strong anti-interference performance from competing metal ions and oxyanions. Environmental sample testing further validated its applicability, showing high recovery rates (90.3–109.8 %). The results showed excellent agreement with the inductively coupled plasma mass spectrometry (ICP-MS) measurements as a conventional Hg(II) detection method. This work advances DNA-enzyme-mediated electrochemical sensing by integrating molecular recognition with an intrinsic detoxification mechanism. The findings provided a promising foundation for the next-generation bioelectronic platforms, enabling real-time monitoring of both inorganic mercury and selenate and a potential mercury removal method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


