改写赖氨酸反应性:赖氨酸靶向生物偶联,通过仿生极性反转实现多种生物分子修饰
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在这里,我们提出了一种生物启发的氧化脱胺策略,该策略逆转了赖氨酸反应性的极性,从而允许赖氨酸在肽和蛋白质中的生物偶联,具有前所未有的生物相容性和化学选择性。原位生成的醛中间体促进了多种下游转化,包括15N/ 18o标记,还原胺化,Pinnick氧化和Wittig, Seyferth-Gilbert和Van Leusen反应。为了证明我们的策略的广泛适用性,我们成功地将多种功能有效载荷偶联到semaglutide,全长蛋白和治疗性抗体的主干上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
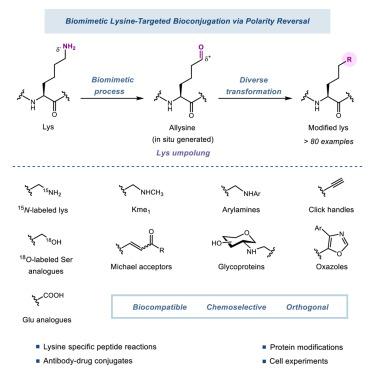
Rewriting lysine reactivity: Lysine-targeted bioconjugation via biomimetic polarity reversal for diversified biomolecule modification
Here, we present a bioinspired oxidative deamination strategy that reverses the polarity of lysine reactivity and thus allows for lysine bioconjugation in peptides and proteins with unprecedented biocompatibility and chemoselectivity. The in-situ-generated aldehyde intermediates facilitate versatile downstream transformations, including 15N/18O-labeling, reductive amination, Pinnick oxidation, and Wittig, Seyferth-Gilbert, and Van Leusen reactions. To demonstrate the broad applicability of our strategy, we successfully conjugated diverse functional payloads onto the backbone of semaglutide, full-length proteins, and therapeutic antibodies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


