通过维可牢样密度依赖靶向肿瘤相关碳水化合物抗原的安全免疫抑制耐药泛癌症免疫治疗
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
双特异性抗体和嵌合抗原受体T细胞是临床上使用的一些最有效的癌症免疫疗法,但大多数癌症的靶向性仍然很差。高亲和力抗体需要最大限度地杀死检测正常组织中的低抗原表达,冒着“靶向,非癌症”毒性。这迫使鉴定癌症限制性细胞表面蛋白抗原,这是罕见的。肿瘤相关碳水化合物抗原(TACAs)是已知的最丰富和最广泛的癌症抗原,但抗体的靶向性很差。在这里,我们描述了甘聚糖依赖性T细胞招募者(GlyTR)泛癌症免疫疗法,利用高亲和力的“维可牢样”凝集素结合来杀死高但不低表达TACA的细胞。GlyTR1和GlyTR2分别结合免疫抑制β1,6 glcnac支链n-聚糖或多种TACAs (Tn, sialyl-Tn, LacDiNAc和GD2),克服肿瘤微环境中的免疫抑制机制,触发靶密度依赖性T细胞介导的泛癌杀伤,但它们在具有类似人的TACA表达的小鼠中缺乏毒性。与TACAs结合的密度依赖性凝集素提供了高效和安全的泛癌症免疫治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
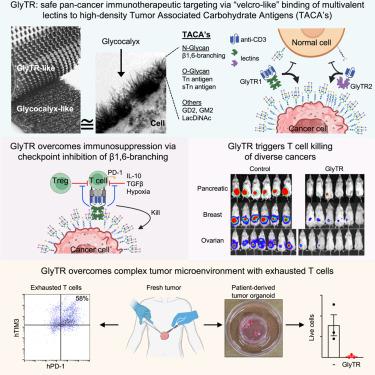
Safe immunosuppression-resistant pan-cancer immunotherapeutics by velcro-like density-dependent targeting of tumor-associated carbohydrate antigens
Bispecific antibodies and chimeric antigen receptor T cells are some of the most potent cancer immunotherapeutics in clinical use, yet most cancers remain poorly targetable. High-affinity antibodies required to maximize killing detect low antigen expression in normal tissue, risking “on-target, off-cancer” toxicity. This compels identification of cancer-restricted cell-surface protein antigens, which are rare. Tumor-associated carbohydrate antigens (TACAs) are the most abundant and widespread cancer antigens known but are poorly targetable by antibodies. Here, we describe glycan-dependent T cell recruiter (GlyTR) pan-cancer immunotherapeutics that utilize high-avidity “velcro-like” lectin binding to kill cells with high but not low TACA expression. GlyTR1 and GlyTR2 bind immunosuppressive β1,6GlcNAc-branched N-glycans or multiple TACAs (Tn, sialyl-Tn, LacDiNAc, and GD2), respectively, overcome immunosuppressive mechanisms in the tumor microenvironment and trigger target-density-dependent T cell-mediated pan-cancer killing, yet they lack toxicity in mice with human-like TACA expression. Density-dependent lectin binding to TACAs provides highly potent and safe pan-cancer immunotherapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


