微生物群通过保护卵巢储备来延长小鼠的生殖寿命
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
六分之一的人患有不孕症,但其潜在机制尚不清楚。我们表明,微生物群控制着雌性小鼠的生殖寿命。无菌小鼠的原始卵泡减少,闭锁增加,卵巢纤维化,导致产仔少,后代少,生殖寿命短。无菌小鼠出生时具有类似的卵巢储备,但在出生后发育过程中表现出过度激活、进展受损和闭锁增加。微生物定植在一个关键的产后窗口挽救卵巢储备的损失通过正常化卵泡动力学和基因表达模式。这些变化与短链脂肪酸(SCFA)增加平行,SCFA管理减轻无菌小鼠卵巢功能障碍。类似的卵母细胞功能障碍发生在喂食高脂肪饮食的常规饲养小鼠中,但额外的膳食纤维有助于保持卵母细胞质量和胚胎能力。因此,宿主-微生物的相互作用塑造了女性的生育能力,微生物群靶向干预可能为解决生殖障碍提供策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
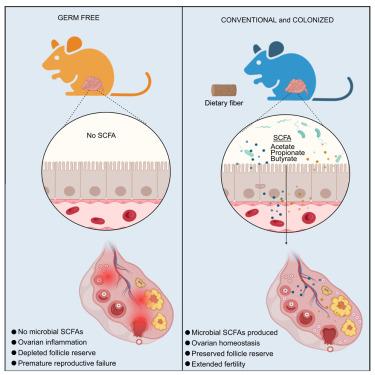
The microbiota extends the reproductive lifespan of mice by safeguarding the ovarian reserve
Infertility affects one in six people, but the underlying mechanisms remain unclear. We show that the microbiota governs female reproductive longevity in mice. Germ-free mice have fewer primordial follicles, increased atresia, and ovarian fibrosis, leading to smaller litters, fewer offspring, and a shorter reproductive lifespan. Germ-free mice are born with a similar ovarian reserve but display excessive activation, impaired progression, and increased atresia during post-natal development. Microbiome colonization during a critical post-natal window rescues premature ovarian reserve loss by normalizing follicle kinetics and gene expression patterns. These changes parallel increased short-chain fatty acids (SCFAs), and SCFA administration mitigates ovarian dysfunction in germ-free mice. Similar oocyte dysfunction occurred in conventionally raised mice fed a high-fat diet, but additional dietary fiber helped preserve oocyte quality and embryo competence. Thus, host-microbe interactions shape female fertility, and microbiota-targeted interventions may offer strategies to address reproductive disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


