共轭聚苯胺微孔中鞣花酸的原位约束及其对汞的高效捕集(ⅱ)
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
在实际废水处理中,微孔聚合物和多酚类材料对重金属离子的吸附性能往往有限,这主要是由于微孔聚合物的孔结构受到限制,而多酚类材料的化学稳定性较差。本文通过在共轭微孔聚苯胺(CMPA)中原位约束鞣花酸(EA),开发了一种新型EA@CMPA复合材料,用于高效去除汞(II)。EA通过双重相互作用(1)氢键和(2)质子化作用锚定在CMPA内。该设计使复合材料具有分层结构的介孔扩散通道和丰富的活性吸附位点,从而提高了EA@CMPA的吸附动力学和吸附容量。所得EA@CMPA(200)对Hg(II)的吸附率h为640 mg g−1 min−1,吸附量为1024mg g−1。值得注意的是,EA@CMPA在经过7次密集重复使用后,再生效率保持在81.5 %以上,这表明水溶性鞣花酸被有效地限制在孔隙通道内。对实际含汞废水也表现出良好的抗干扰能力和选择性,Hg(II)去除率为95.41 %,选择性接近100% %。通过FT-IR, XPS和DFT计算对吸附行为和机理进行了表征,揭示了C=O和- nh -位点之间的协同相互作用驱动了较好的Hg(II)捕获。这项研究强调了EA@CMPA作为一种高效的汞修复吸附剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
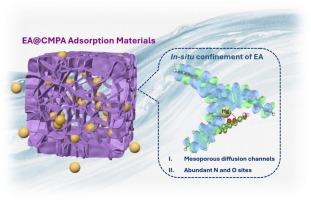
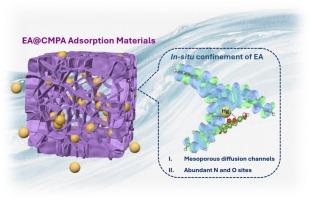
In-situ confinement of ellagic acid within conjugated microporous poly(aniline)s for efficient capture mercury (II)
In practical wastewater treatment, microporous polymers and polyphenolic materials often suffer from limited adsorption performance for heavy metal ions, primarily due to the restricted pore structure of the former and the poor chemical stability of the latter. Herein, a novel EA@CMPA composite is developed via in-situ confinement of ellagic acid (EA) within Conjugated Microporous Poly(aniline) (CMPA) for efficient Hg(II) removal. EA is anchored within CMPA through dual interactions: (i) hydrogen-bonding and (ii) protonation. This design endows the composite with hierarchically structured mesoporous diffusion channels and abundant active adsorption sites, thereby enabling EA@CMPA to enhancement of the adsorption kinetics and capacity. The resulted EA@CMPA(200) has high Hg(II) adsorption rate h of 640 mg g-1 min-1 and adsorption capacity of 1024 mg g-1. Notably, EA@CMPA maintains a regeneration efficiency over 81.5% after 7 cycles of intensive reuse, demonstrating that the water-soluble ellagic acid is efficiently confined within the pore channels. It also exhibits excellent anti-interference ability and selectivity in actual Hg-containing wastewater, with Hg(II) removal rate of 95.41% and selectivity approaching 100%. The adsorption behavior and mechanism are characterized by FT-IR, XPS, and DFT calculations, revealing that the synergistic interactions between C=O and –NH– sites drive superior Hg(II) capture. This study highlights the potential of EA@CMPA as a high-performance adsorbent for mercury remediation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


