工程益生菌乳酸菌包封增强可切换蛋白释放
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
活微生物疗法承诺在疾病部位进行精确的、可编程的干预,然而大多数按需药物释放的演示仍然依赖于大肠杆菌,其丰富的基因工具箱在益生菌中是无与伦比的。特别是在乳酸菌等非模式益生菌中,严重缺乏调节原位蛋白生产的遗传部分。在这里,我们为植物乳酸杆菌益生菌配备了高性能的遗传开关,并展示了材料封装如何进一步增强它们的行为。通过将植物L. plantarum (Ptec)中最强的组成启动子(cumate or vanilla -responsive operators and repressor)与cumate或vanilla -responsive operators结合,我们生成了两个支持微摩尔范围感应的开关。在快速生长的培养条件下,观察到与酸化相关的开关泄漏,这可能会影响其精确按需输送药物的适用性。此外,这种泄漏也限制了这些工程益生菌可以可靠使用的持续时间。通过温和的温度或营养限制来限制生长,可以抑制酸化和渗漏。引人注目的是,将工程细胞固定在核壳海藻酸盐珠(重组乳酸杆菌,珍珠蛋白洗脱海藻酸盐)中几乎消除了渗漏,实现了对细胞内报告细胞和分泌酶的日规模可逆控制。当两个携带正交开关的菌株被共封装时,甚至在小型化到亚毫米微珠后,这种泄漏抑制仍然存在。这些结果扩大了植物乳杆菌的遗传工具箱,证明了遗传电路设计和材料封装之间的协同作用,并将乳酸菌推向刺激响应性治疗平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
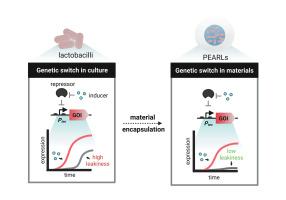
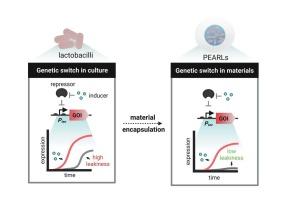
Encapsulation-enhanced switchable protein release from engineered probiotic lactobacilli
Living microbial therapeutics promise precise, programmable interventions at disease sites, yet most demonstrations of on demand drug release still rely on Escherichia coli, whose rich genetic toolkit is unmatched among probiotics. In particular, genetic parts to regulate in situ protein production are severely lacking in non-model probiotic bacteria like lactobacilli. Here, we equip the probiotic Lactiplantibacillus plantarum with high-performance genetic switches and show how material encapsulation can further enhance their behavior. By integrating cumate or vanillate-responsive operators and repressors with the strongest constitutive promoter in L. plantarum (Ptec), we generated two switches that support micromolar range induction. In rapidly growing culture conditions, acidification-associated leakiness of the switch was observed, which could compromise their applicability for precise on-demand delivery of drugs. Furthermore, such leakiness also limits the duration for which these engineered probiotics can be reliably used. By restricting growth through mild temperature or nutrient limitation, acidification and leakiness were suppressed. Strikingly, immobilizing the engineered cells in core-shell alginate beads (Protein Eluting Alginate with Recombinant Lactobacilli, PEARLs) almost eliminated leakiness, enabling day-scale, reversible control of intracellular reporters and secreted enzymes. This leakiness suppression persisted when two strains carrying orthogonal switches were co-encapsulated and even after miniaturization to submillimeter beads. These results expand the genetic toolbox of probiotic L. plantarum, demonstrate the synergy between genetic circuit design and material encapsulation, and advance lactobacilli toward stimuli-responsive therapeutic platforms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


