羟氯喹功能化的可电离脂质减轻mRNA治疗中的炎症反应
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
基于脂质纳米颗粒(LNP)的mRNA治疗方法,特别是SARS-CoV-2疫苗的成功,面临着由可电离脂质引起的炎症的挑战。这些可电离的脂质可以激活免疫系统,特别是当与核酸共同递送时,导致不良的炎症反应。我们介绍了一类以羟氯喹(HCQ)功能化的新型抗炎电离脂质,它可以抑制脂质诱导和核酸诱导的免疫激活。这些hcq功能化LNPs (HL LNPs)在保持有效mRNA传递的同时表现出降低的促炎反应。结构和物理化学分析表明,hcq功能化导致颗粒结构不同,稳定性显著提高。HL LNPs的有效性在各种治疗环境中得到了证明,包括针对水痘带状疱疹病毒(VZV)的预防性疫苗接种模型和靶向PCSK9的CRISPR-Cas9基因编辑。值得注意的是,在重复给药后,HL LNPs显示出强大的mRNA表达,解决了炎症问题并确保了持续的治疗效果。这些发现强调了hcq功能化LNPs在扩大mRNA治疗安全使用方面的潜力,特别是在需要重复给药和必须最小化炎症引起的副作用的应用中。本文章由计算机程序翻译,如有差异,请以英文原文为准。

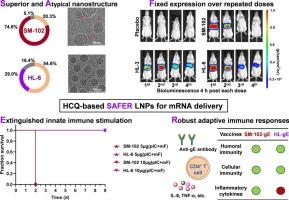
Hydroxychloroquine-functionalized ionizable lipids mitigate inflammatory responses in mRNA therapeutics
Lipid nanoparticle (LNP)-based mRNA therapeutics, highlighted by the success of SARS-CoV-2 vaccines, face challenges due to inflammation caused by ionizable lipids. These ionizable lipids can activate the immune system, particularly when co-delivered with nucleic acids, leading to undesirable inflammatory responses. We introduce a novel class of anti-inflammatory ionizable lipids functionalized with hydroxychloroquine (HCQ), which suppresses both lipid-induced and nucleic acid-induced immune activation. These HCQ-functionalized LNPs (HL LNPs) exhibit reduced proinflammatory responses while maintaining efficient mRNA delivery. Structural and physicochemical analyses revealed that HCQ-functionalization results in a distinct particle structure with significantly improved stability. The efficacy of HL LNPs was demonstrated across various therapeutic contexts, including a prophylactic vaccination model against varicella-zoster virus (VZV) and CRISPR-Cas9 gene editing targeting PCSK9. Notably, HL LNPs showed robust mRNA expression after repeated administration, addressing concerns of inflammation and ensuring sustained therapeutic effects. These findings highlight the potential of HCQ-functionalized LNPs in expanding the safe use of mRNA therapeutics, particularly for applications requiring repeated dosing and in scenarios where inflammation-induced side effects must be minimized.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


