邻苯二胺转酰胺的机械化学解锁:n -取代邻苯二胺的途径
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
据报道,邻苯二甲酸亚胺与伯和仲脂肪胺的转酰胺(氨解)反应性由机械力诱导,不需要催化剂、酸或热活化。该反应在室温下进行,主要产物为n取代的邻苯二胺。它是在一个使用不锈钢罐和球的混合机中进行的。该范围通过20多个例子进行了演示,实现了高达98%的收率。本文章由计算机程序翻译,如有差异,请以英文原文为准。

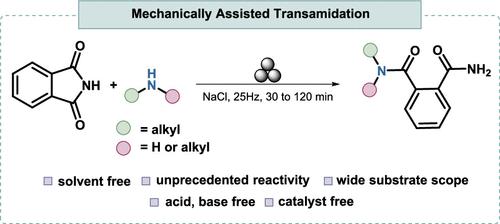
Mechanochemical Unlocking of Phthalimide Transamidation: A Path to N-Substituted Phthalamides
The transamidation (aminolysis) of phthalimide with primary and secondary aliphatic amines is reported, where reactivity is induced by mechanical forces without the need for catalysts, acids, or thermal activation. The reaction proceeds at room temperature to afford N-substituted phthalamides as the major products. It is carried out in a mixer mill using stainless-steel jars and balls. The scope is demonstrated with more than 20 examples, achieving yields of up to 98%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


