通过分泌蛋白酶活性测定评估癌细胞的单细胞异质性和侵袭潜力
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本文介绍了一种在微流控阵列室中分离的单个癌细胞分泌蛋白酶活性的测定方法。该方法建立在双向电泳和双极(DEP-BPE)平台上,能够在选择性、无标记的基于dep的细胞分离后进行活细胞分泌分析。虽然许多现有的检测依赖于群体水平的测量或标记策略,但仍然迫切需要无标记的功能工具,以捕获单细胞水平活细胞分泌行为的异质性。结果报告的工作流程可以量化单个细胞之间的蛋白酶分泌行为,获取时间分泌谱,并鉴定高侵袭性细胞。具体而言,我们评估了分离癌细胞的基质金属蛋白酶9 (MMP9)分泌,并报告了单细胞水平上MMP9分泌浓度和分泌动态的实质性异质性。线性荧光响应(R2 = 0.98)可在280 pL体积中定量到3.31 nM的MMP9 (5.4 × 105分子)。在混合群体中,132个细胞中有34个(26%)分泌高于背景的MMP9, 14个细胞分泌比任何未经治疗的对照细胞都多,揭示了一个高度侵袭性的亚群体。该平台实现了侵袭性潜能的单细胞功能分析,为评估疾病进展和治疗反应提供了细胞异质性的见解。这项工作的新颖之处在于它将DEP的单细胞分离与分泌酶的功能测定相结合,DEP对细胞类型有选择性。该方法可推广到许多细胞类型的选择性分离和分子分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
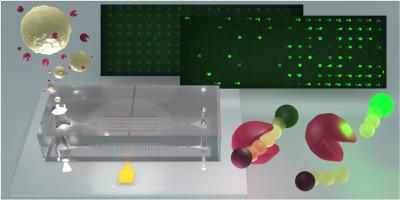
Evaluation of single-cell heterogeneity and invasive potential in cancer cells via secreted protease activity assay
Background
This paper presents an assay for the secreted protease activity of single cancer cells isolated within chambers of a microfluidic array. The methodology builds on the dielectrophoresis and bipolar electrode (DEP-BPE) platform, enabling live-cell secretion analysis following selective, label-free DEP-based cell isolation. While many existing assays rely on population-level measurements or labeling strategies, there remains a critical need for label-free, functional tools that capture heterogeneity in live-cell secretion behavior at the single-cell level.
Results
The reported workflow allows for quantification of protease secretion behavior among individual cells, acquisition of temporal secretion profiles, and identification of highly invasive cells. Specifically, we assess the matrix metalloprotease 9 (MMP9) secretion of isolated cancer cells and report substantial heterogeneity in both secreted MMP9 concentration and secretion dynamics at the single-cell level. A linear fluorescence response (R2 = 0.98) enables quantification down to 3.31 nM MMP9 (5.4 × 105 molecules) in 280 pL volumes. In a mixed population, 34 of 132 cells (26 %) exhibited MMP9 secretion above background, and 14 cells secreted more than any untreated control cell, revealing a highly invasive subpopulation obscured in ensemble measurements.
Significance
This platform enables functional single-cell analysis of invasive potential, providing insights into cellular heterogeneity that are critical for evaluating disease progression and treatment response. The novelty of this work lies in its integration of single-cell isolation by DEP, which is selective for cell type, with a functional assay for a secreted enzyme. The approach is generalizable to selective isolation and molecular assay of many cell types.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


