CuCo复合氧化物协同催化:电子结构工程提高甲醇燃料电池的双功能活性
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
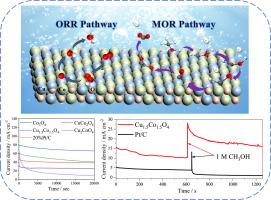
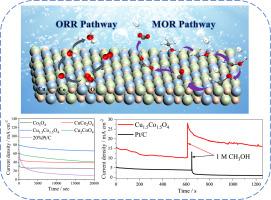
直接甲醇燃料电池(dmfc)以液态甲醇为燃料,具有6.13 kWh/kg的高能量密度和低污染排放,被广泛认为是理想的“绿色”能源转换器。然而,在甲醇氧化反应(MOR)和氧还原反应(ORR)中,电催化剂受到甲醇脱氢中间体(CO)的毒害,抑制了电子传递,减慢了反应动力学。因此,开发一种高性能、耐co双功能催化剂是实现dmfc高效稳定运行的关键。本研究通过双金属复合氧化物相互作用产生协同活性位点,从而提高电子传递效率,优化MOR和ORR工艺。实验结果表明,Cu1.5Co1.5O4催化剂具有明显的改进空间,其起始电位(Eonset = 0.857 V)和半波电位(E1/2 = 0.746 V)达到Pt/C催化剂的83%。具有75.76 mA cm-2的电流密度和优异的CO电阻。在dmfc中,其具有竞争力的功率密度为20.45 mW cm-2。DFT计算证实复合氧化物能有效调节*CO和*OH中间体结合能,同时显著降低ORR理论中的过电位。通过d-和p-带中心理论进一步验证了吸附强度的变化。这为CuCo复合氧化物在dmfc技术和清洁能源系统中的应用提供了新的设计原则。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CuCo composite oxide synergistic catalysis: Electronic structure engineering boosts bifunctional activity for methanol fuel cells
Direct methanol fuel cells (DMFCs), which utilize liquid methanol as fuel, exhibit a high energy density of 6.13 kWh/kg and low pollution emissions, are widely regarded as ideal "green" energy converters. However, electrocatalysts are poisoned by methanol dehydrogenation intermediates (CO) during methanol oxidation reaction (MOR) and oxygen reduction reaction (ORR), which inhibits electron transfer and slows reaction kinetics. Therefore, developing a high-performance, CO-tolerant bifunctional catalyst is crucial for achieving efficient and stable operation of DMFCs. In this study, synergistic active sites were generated through interaction of bimetallic composite oxides, thereby enhancing electron transfer efficiency and optimizing both MOR and ORR processes. Experimental results demonstrate that Cu1.5Co1.5O4 catalyst shows significant room for improvement, with its onset potential (Eonset = 0.857 V) and half-wave potential (E1/2 = 0.746 V) reaching 83 % of Pt/C catalysts. Demonstrating an outstanding current density of 75.76 mA cm-2 coupled with superior CO resistance. In DMFCs, it delivered a competitive power density of 20.45 mW cm-2. DFT calculations confirmed that composite oxide effectively regulates the binding energies of *CO and *OH intermediates, while notably reducing the overpotential in ORR theory. The changes in adsorption strength were further validated by d- and p-band center theory. This provides new design principles for the application of CuCo compositew oxides in DMFCs technology and clean energy systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


