针对脂质II周期和细菌膜对抗多药耐药的广谱阿比烷二萜衍生物
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
耐药超级细菌的威胁不断升级,迫切需要开发具有新型支架和机制的抗生素。本文以鼠尾草酸(CA)为原料,采用“胺/胍”修饰策略,合理设计合成了新型的阿比烷二萜衍生物。大多数设计的衍生物显示出比CA甚至万古霉素更大的活性(~ 2 - 64倍)。优化后的4c具有抗MRSA、VRE、CRAB和分枝杆菌的超广谱活性,同时具有快速杀菌能力、低耐药发展倾向和良好的安全性。机制研究首次揭示了一种新的独立的和协同的“细胞壁-膜”双重抑制机制,分别通过干扰细菌脂质II周期和诱导膜的透性和去极化。随后的体内研究表明,4c在斑马鱼和小鼠感染模型中表现出良好的疗效。总的来说,这些发现突出了从CA中提取的阿比烷二萜的抗菌潜力,并确定了4c是一种有希望的抗耐药细菌感染的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
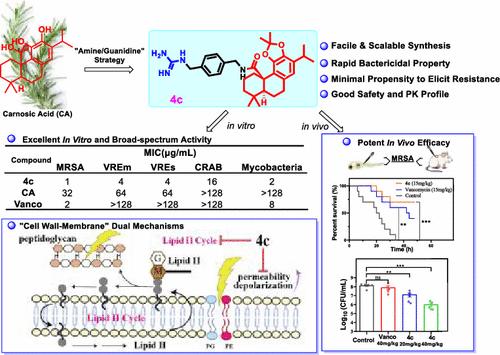
A Broad-Spectrum Abietane Diterpenoid Derivative that Targets Lipid II Cycle and Bacterial Membrane to Combat Multidrug Resistance
The escalating threat of drug-resistant superbugs has urgently necessitated the development of antibiotics with novel scaffolds and mechanisms. Herein, we rationally designed and synthesized novel abietane diterpenoid derivatives from carnosic acid (CA) inspired by the “amine/guanidine” modification strategy. Most derivatives designed exhibited greater activities than CA and even vancomycin (∼2–64-fold). The optimized 4c demonstrated ultrabroad-spectrum activity against MRSA, VRE, CRAB, and mycobacteria, coupled with desirable properties of rapid bactericidal ability, low resistance development propensity, and good safety profile. Mechanistic studies first revealed a novel independent and synergistic “cell wall–membrane” dual inhibitory mechanism, respectively, via interfering with bacterial lipid II cycle and inducing membrane permeabilization and depolarization. Subsequent in vivo studies elucidated that 4c demonstrated favorable efficacy in zebrafish and mouse infection models. Collectively, these findings highlight the antimicrobial potential of abietane diterpenoids derived from CA and identify 4c as a promising drug candidate against drug-resistant bacterial infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


