聚乙二醇-曲前列地尼偶联物在肺动脉高压持续局部给药中的合成及体外评价。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
treprostiil (TRE)是一种前列环素类似物,被批准用于治疗肺动脉高压(PAH)。尽管其有效,但TRE的半衰期短,需要频繁或持续给药以维持治疗水平,同时尽量减少不良反应。为了改善TRE的药代动力学和肺部靶向性,我们设计了一系列吸入用聚乙二醇(PEG)酯缀合物。通过聚合物偶联实现的分子大小的增加阻止了TRE在肺泡上皮上的被动扩散,这是小的亲脂性药物递送到肺部的一个共同缺点。TRE和PEG之间的酯键使活性化合物在肺泡空间内逐渐释放。用7种不同的含炔连接剂对TRE进行了化学修饰,随后通过点击化学偶联得到peg叠氮化物6kda。这些连接剂被精心设计来调节可切割酯键周围的化学环境,从而可以系统地评估对PEG-TRE共轭物稳定性的空间和电子效应。健康大鼠支气管肺泡灌洗(BAL)中的药物释放研究表明,空间阻碍和电子稳定的连接物显著减缓了TRE的释放。此外,缀合物的稳定性依赖于酶的可用性,较高的缀合物-酶比导致较慢的释放,表明酶饱和是控制药物释放的潜在机制。总的来说,这些发现证明了一种可调整的策略,可以从生物介质中的聚合物药物系统中释放药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
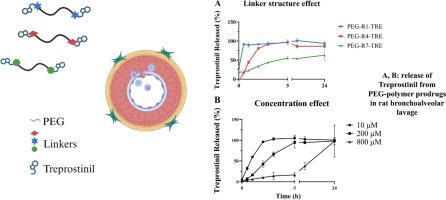
Synthesis and in vitro evaluation of polyethylene glycol-treprostinil conjugates for sustained local delivery in pulmonary arterial hypertension
Treprostinil (TRE) is a prostacyclin analogue approved for the treatment of pulmonary arterial hypertension (PAH). Despite its effectiveness, TRE has a short half-life, necessitating frequent or continuous administration to maintain therapeutic levels while minimizing adverse effects. To improve pharmacokinetics and pulmonary targeting of TRE, we designed a series of polyethylene glycol (PEG) ester conjugates for inhalation. The increase in molecular size achieved through polymer conjugation prevents the passive diffusion of TRE across the alveolar-capillary barrier, a common drawback of small, lipophilic drugs delivered to the lungs. The ester bond between TRE and PEG enables a gradual release of the active compound within the alveolar space. TRE was chemically modified with seven different alkyne-bearing linkers and subsequently conjugated to PEG-azide 6 kDa via click chemistry. These linkers were strategically designed to modulate the chemical environment around the cleavable ester bond, allowing for the systematic evaluation of the steric and electronic effects on the stability of the PEG-TRE conjugates. Drug release studies in bronchoalveolar lavage from healthy rats demonstrated that sterically hindered and electronically stabilized linkers significantly slowed the release of TRE. Moreover, conjugate stability was dependent on the enzymes availability, with a higher conjugate-enzyme ratio leading to slower release, suggesting enzyme saturation as a potential mechanism for controlled drug release. Overall, these findings demonstrate a tunable strategy for releasing drugs from polymer-drug conjugates in biological media.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


