Rh/γ-Al2O3催化二甲基甲苯-2,4-二氨基甲酸酯选择性加氢:机理和动力学
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
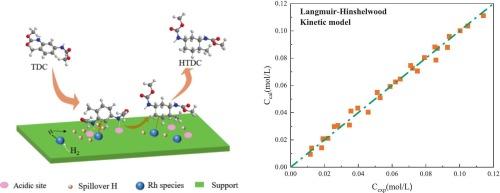
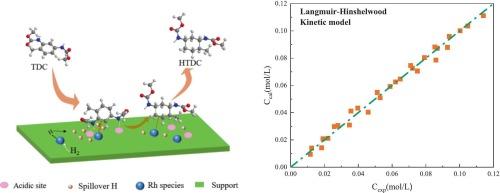
二甲基甲苯-2,4-二氨基甲酸酯(TDC)选择性加氢合成甲基环己基-2,4-二氨基甲酸酯(HTDC)是绿色合成甲基环己烷-2,4-二异氰酸酯(HTDI)的重要步骤。然而,由于反应机理不明确,反应动力学缺乏,阻碍了加氢工艺的商业化发展。本文研究了TDC在Rh/γ-Al2O3催化剂上的选择性加氢反应,重点研究了反应机理和动力学。首先,采用原位漂移技术研究了反应组分在Rh/γ-Al2O3催化剂表面的吸附行为。结果表明,TDC和氢在Rh表面的吸附是竞争的,但HTDC的吸附比TDC弱得多。在此基础上,基于Langmuir-Hinshelwood机理对TDC选择性加氢动力学进行了探讨。采用混合算法优化动力学参数,通过统计判别选择最优模型,并与DRIFTS分析结果进行一致性验证。在苯环上加成第一个氢原子的表面反应被确定为速率决定步骤。在此基础上,建立了Rh/γ-Al2O3催化TDC选择性加氢反应动力学,活化能为48.1 kJ·mol−1。该工作将为HTDI合成绿色路线的发展,特别是TDC选择性加氢制HTDC工艺提供技术参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rh/γ-Al2O3 catalyzed selective hydrogenation of dimethyl toluene-2,4-dicarbamate: mechanism and kinetics
The selective hydrogenation of dimethyl toluene-2,4-dicarbamate (TDC) to methyl cyclohexyl-2,4-dicarbamate (HTDC) is an essential step for the green synthesis of methylcyclohexane-2,4-diisocyanate (HTDI). However, an unclear reaction mechanism and a lack of its reaction kinetics hamper the commercial development of the hydrogenation process. In this work, we investigated the selective hydrogenation of TDC over Rh/γ-Al2O3 catalyst and focused on the reaction mechanism and kinetics. Firstly, the adsorption behavior of the reaction components on the surface of Rh/γ-Al2O3 catalyst was studied using in situ DRIFTS technique. It was revealed that TDC and hydrogen are competitively adsorbed on the Rh surface but HTDC is adsorbed much more weakly compared with TDC. On this basis, the kinetics of TDC selective hydrogenation were probed based on the Langmuir-Hinshelwood mechanism. After optimizing kinetic parameters via a hybrid algorithm and selecting the optimal model through statistical discrimination, its consistency was confirmed with DRIFTS analysis results. The surface reaction involving the addition of the first hydrogen atom to the benzene ring was identified as the rate-determining step. Based on these, the kinetics of TDC selective hydrogenation catalyzed by Rh/γ-Al2O3 were established with an activation energy of 48.1 kJ·mol−1. This work will provide a technical reference for the development of green route to HTDI synthesis, especially the selective hydrogenation of TDC to HTDC process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


