Msx2在外显子调控中的作用:在从毛发脱落到再生的过渡过程中调节干细胞生态位
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
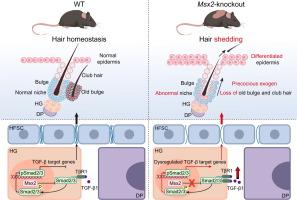
毛囊周期的外生阶段,在此期间,毛囊脱落和新头发出现,代表了一个关键的过渡阶段,涉及明显的结构重塑和粘附变化。尽管其具有重要的基础意义,但调控外生因子的分子机制在很大程度上仍然未知。目的探讨基因Msx2在外显期间调控头发锚定、毛囊干细胞(HFSC)谱系承诺和细胞外基质(ECM)重塑中的作用。方法采用msx2敲除(KO)小鼠,大量RNA测序和晚期外显子转录组比较来鉴定差异表达基因(DEGs)和中断途径。此外,还进行了蛋白质相互作用试验以进行功能研究。结果smsx2缺陷小鼠在随后的毛发周期中仍形成含有HFSCs的新凸起,但这导致旧凸起和棒状毛发锚定失败,导致毛发保留异常和毛发脱落加速。转录组学分析揭示了广泛的转录重编程,特别是在ecm相关基因中,Msx2-KO HFSCs和晚期外显子deg之间存在显著的重叠。Msx2-KO小鼠表现出粘附分子下调,HFSC生态位完整性被破坏,角化增加,HFSC和毛生殖细胞分化异常。机制上,MSX2直接与SMAD蛋白相互作用,调控TGF-β信号,从而调节ECM基因表达,抑制HFSC跨表皮分化。结论我们的研究结果表明Msx2是外显子控制的主要调控因子,协调毛发脱落、HFSC生态位稳定性和ECM重塑。这项研究为毛囊循环动力学提供了新的见解,并确定了Msx2作为促进头发再生和减轻脱发表型的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Roles of Msx2 in exogen control: modulating the stem cell niche during the transition from hair shedding to regeneration
Introduction
The exogen phase of the hair follicle cycle, during which club hairs are shed and new hairs emerge, represents a critical transitional step involving distinct structural remodeling and adhesive changes. Despite its fundamental importance, the molecular mechanisms orchestrating exogen remain largely unknown.Objective
To investigate the role of Msx2 in regulating hair anchoring, hair follicle stem cell (HFSC) lineage commitment, and extracellular matrix (ECM) remodeling during exogen.Methods
We employed Msx2-knockout (KO) mice, bulk RNA sequencing, and late exogen transcriptome comparisons to identify differentially expressed genes (DEGs) and disrupted pathways. Additionally, protein interaction assays were conducted for functional studies.Results
Msx2 deficiency mice still form new bulges containing HFSCs for subsequent hair cycles, but this resulted in the failed anchoring of older bulges and club hairs, leading to aberrant hair retention and accelerated hair shedding. Transcriptomic analysis revealed widespread transcriptional reprogramming, particularly in ECM-related genes, with significant overlap between Msx2-KO HFSCs and late exogen DEGs. Msx2-KO mice exhibited downregulated adhesion molecules, disrupted HFSC niche integrity, increased keratinization, and aberrant differentiation in HFSC and hair germ cells. Mechanistically, MSX2 directly interacts with SMAD proteins to regulate TGF-β signaling, thereby modulating ECM gene expression and suppressing HFSC trans-epidermal differentiation.Conclusions
Our findings establish Msx2 as a master regulator of exogen control, orchestrating hair shedding, HFSC niche stability, and ECM remodeling. This study provides new insights into hair follicle cycling dynamics and identifies Msx2 as a potential therapeutic target for promoting hair regeneration and mitigating alopecia phenotypes.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


