母体CENP-C恢复哺乳动物受精卵的着丝粒对称性,以确保适当的染色体分离
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
在后生动物物种中,着丝粒特异性组蛋白变体CENP-A对于准确的染色体分离至关重要,但其在哺乳动物亲本到受精卵转变过程中的调控作用尚不清楚。为了解决这个问题,我们建立了一个揭示性别特异性动态的CENP-A- mscarlet小鼠模型:成熟精子保留了MII卵母细胞中存在的10%的CENP-A水平。然而,这种差异在第一次有丝分裂之前在受精卵中被解决,使用母体遗传的细胞质CENP-A。值得注意的是,父本着丝粒中CENP-A的增加与感知CENP-A不对称或母体染色体的存在无关。相反,CENP-A的均衡依赖于母体CENP-C向父亲着丝粒的不对称募集。母体CENP-A的耗竭减少了两个原核的总CENP-A,但不破坏平衡。相反,母体CENP-C的减少或其二聚化功能的破坏会损害CENP-A的均衡和染色体分离。因此,母体的CENP-C作为一个关键的表观遗传调节因子,在受精时重置着丝粒对称性,以保持基因组的完整性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
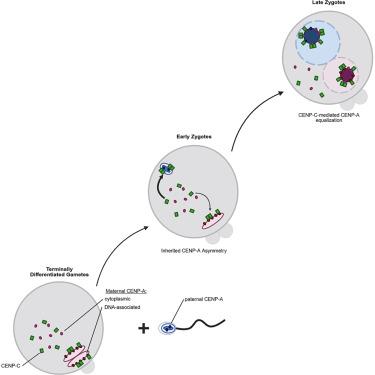
Maternal CENP-C restores centromere symmetry in mammalian zygotes to ensure proper chromosome segregation
Across metazoan species, the centromere-specific histone variant CENP-A is essential for accurate chromosome segregation, yet its regulation during the mammalian parental-to-zygote transition is poorly understood. To address this, we generated a CENP-A-mScarlet mouse model that revealed sex-specific dynamics: mature sperm retain 10% of the CENP-A levels present in MII oocytes. However, this difference is resolved in zygotes prior to the first mitosis, using maternally inherited cytoplasmic CENP-A. Notably, the increase in CENP-A at paternal centromeres is independent of sensing CENP-A asymmetry or the presence of maternal chromosomes. Instead, CENP-A equalization relies on the asymmetric recruitment of maternal CENP-C to paternal centromeres. Depletion of maternal CENP-A decreases total CENP-A in both pronuclei without disrupting equalization. In contrast, reducing maternal CENP-C or disruption of its dimerization function impairs CENP-A equalization and chromosome segregation. Therefore, maternal CENP-C acts as a key epigenetic regulator that resets centromeric symmetry at fertilization to preserve genome integrity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


