优化非晶多药配方:喷雾干燥颗粒工程方法。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
尽管在多药制剂领域取得了进展,但开发和制造多药制剂仍然面临着巨大的挑战,特别是对于低水溶性药物。在这里,这一关键问题是解决工程无定形多药配方与优化性能的吸收部位使用喷雾干燥技术。含有阿扎那韦和利托那韦的制剂,单独或联合,通过喷雾干燥生产。优化了水溶液中赋形剂的含量,制备了粒径可控的稳定的非晶颗粒进料悬浮液。采用粉末x射线衍射(PXRD)、热分析、激光衍射和扫描电镜(SEM)对粉末配方进行了表征。测定药物含量,并进行溶出度研究。采用动态光散射法测定了饲料悬浮液中和粉末溶解后胶体相的粒径。在25°C/ 60% RH和40°C/ 75% RH条件下进行了为期4周的稳定性研究。DSC和PXRD证实该配方为非晶态。喷雾干燥制剂中药物含量为98 ~ 108%。激光衍射测得颗粒直径在5 ~ 10µm之间,扫描电镜显示颗粒表面有褶皱和不规则形状。联合制剂溶解后形成的胶体相的粒径在900 nm处稳定,持续时间120 min。在整个稳定性研究期间,在两种研究条件下,配方都保持无定形。这些发现突出了颗粒工程的潜力,其中机械信息选择赋形剂与适当的喷雾干燥过程相结合,以实现高度稳定和坚固的无定形多药配方-这对于确保有效的药物性能和患者治疗至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
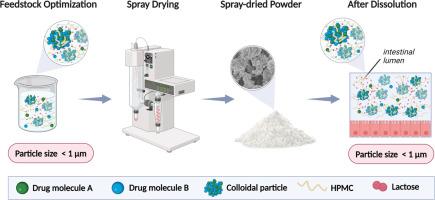
Optimizing amorphous multidrug formulations: A particle engineering approach through spray drying
Despite advances in the field of multidrug formulations, developing and manufacturing them still poses substantial challenges, particularly for drugs with low aqueous solubility. Here, this critical issue was addressed by engineering amorphous multidrug formulations with optimized performance at the site of absorption using the spray drying technique. Formulations containing atazanavir and ritonavir, alone or in combination, were produced by spray drying. Excipient content in aqueous solution was optimized to generate a stable feed suspension of amorphous particles with controlled particle size. The powder formulations were characterized by powder X-ray diffraction (PXRD), thermal analysis, laser diffraction, and scanning electron microscopy (SEM). The drug content was assayed, and a dissolution study was performed. Dynamic light scattering was used to measure particle size of the colloidal phase in the feed suspension and after dissolution of powder. A stability study was conducted at 25 °C/60 % RH and 40 °C/75 % RH condition for 4 weeks. DSC and PXRD confirmed the formulations to be amorphous. Drug content in the spray-dried formulations ranged from 98 to 108 %. Laser diffraction measured the particles to be from 5–10 µm and SEM showed they had wrinkled and irregularly shaped surfaces. The particle size of the colloidal phase formed upon dissolution of combination formulation was stable at 900 nm over 120 min. The formulations remained amorphous under both studied conditions throughout the stability study period. These findings highlight the potential of particle engineering, where a mechanistically informed selection of excipients is combined with an appropriate spray-drying process, to achieve highly stable and robust amorphous multidrug formulations —critical for ensuring effective drug performance and patient treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


