膜曲率控制氧化磷酸化系统的效率
IF 1.4
Q4 CELL BIOLOGY
Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology
Pub Date : 2025-06-13
DOI:10.1134/S1990747825700059
引用次数: 0
摘要
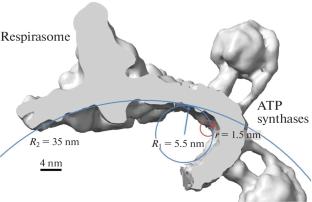
本文分析了线粒体的结构和功能组织。研究表明,线粒体呼吸链和ATP合酶二聚体的超络合物引起膜脂的变形导致它们相互吸引。研究表明,质子泵附近的膜弯曲为质子向ATP合酶的运动创造了一个专用的方向。线粒体可以在松散耦合的备用模式下工作,但也具有低氧化损伤。在高能量需求条件下,由于超微结构的改变,它们可以切换到氧化磷酸化系统(OXPHOS)的紧密耦合和聚类模式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Membrane Curvature Controls the Efficiency of Oxidative Phosphorylation System
This paper analyzes the structural and functional organization of mitochondria. It is shown that deformation of membrane lipids by supercomplexes of the mitochondrial respiratory chain and ATP synthase dimers leads to their mutual attraction. It has been shown that membrane bending near the proton pump creates a dedicated direction for the movement of protons to ATP synthase. Mitochondria can operate in the standby mode with loose coupling but also with low oxidative damage. They can switch to the mode of tight coupling and clustering of oxidative phosphorylation system (OXPHOS) at high energy demand conditions due to the changes in ultrastructure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
0.00%
发文量
28
期刊介绍:
Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology is an international peer reviewed journal that publishes original articles on physical, chemical, and molecular mechanisms that underlie basic properties of biological membranes and mediate membrane-related cellular functions. The primary topics of the journal are membrane structure, mechanisms of membrane transport, bioenergetics and photobiology, intracellular signaling as well as membrane aspects of cell biology, immunology, and medicine. The journal is multidisciplinary and gives preference to those articles that employ a variety of experimental approaches, basically in biophysics but also in biochemistry, cytology, and molecular biology. The journal publishes articles that strive for unveiling membrane and cellular functions through innovative theoretical models and computer simulations.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


