粉碎动力学约束:用于原位mRNA成像和精确癌症治疗的分层激活DNAzyme纳米天线
IF 10.5
1区 生物学
Q1 BIOPHYSICS
引用次数: 0
摘要
癌症的进展是复杂的事件,涉及不同rna的异常表达。基于dnazyme的成像引导基因治疗为有效治疗严重疾病提供了良好的前景,但受到无条件激活、传递效率低、扩增能力有限的限制。在此,我们构建了粉碎动力学约束:可分层激活的DNAzyme纳米天线,用于原位mRNA成像和精确的癌症治疗。该纳米系统包含多功能模块,包括适体介导的肿瘤靶标模块,i-motif门控识别模块,金属离子激活扩增模块和反义寡核苷酸(ASO)诱导的治疗模块。一旦遇到癌细胞,发夹1 (H1)通过适体AS1411识别核蛋白,同时H1中的i-motif在细胞外酸性pH下进行结构转换,使H2, H3, H4, H5交叉打开,用于双面DNAzyme纳米天线的原位自组装。在被癌细胞内化后,纳米天线中的DNAzymes以细胞内Mg2+作为辅助因子裂解底物,释放出大量含有ASOs的分子信标。ASOs与Egr-1 mRNA杂交,诱导Cy5荧光恢复,用于Egr-1 mRNA成像和沉默。该纳米天线的检测限为7.46 fM。它可以分析不同人类细胞中Egr-1 mRNA的水平,并区分乳腺癌组织和健康组织中Egr-1 mRNA的水平。此外,它还可用于体内Egr-1 mRNA的高对比度成像、有效的基因沉默和增强肿瘤消融,在智能基因治疗和精确纳米药物方面具有广阔的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
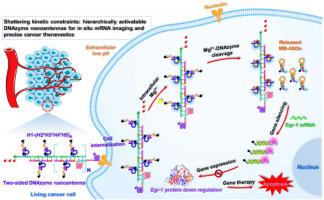
Shattering kinetic constraints: hierarchically activatable DNAzyme nanoantennas for in situ mRNA imaging and precise cancer theranostics
Cancer progressions are complicated events with involvement of abnormal expression of different RNAs. DNAzyme-based imaging-guided gene therapy offers promising prospects for effectively treating serious diseases, but it is constrained by unconditional activation, inefficient delivery, and limited amplification capacity. Herein, we construct shattering kinetic constraints: hierarchically activatable DNAzyme nanoantennas for in situ mRNA imaging and precise cancer theranostics. This nanosystem incorporates multifunctional modules including an aptamer-mediated tumor-target module, an i-motif-gated recognition module, a metal ion-activated amplification module, and an antisense oligonucleotide (ASO)-induced therapy module. Once encountering cancer cells, hairpin 1 (H1) recognizes the nucleolin via an aptamer AS1411, and meanwhile the i-motif in H1 undergoes structure switching at extracellular acid pH, enabling the cross-opening of H2, H3, H4, H5 for in situ self-assembly of two-sided DNAzyme nanoantennas. Upon internalization by cancer cells, DNAzymes in the nanoantenna cleave the substrates with intracellular Mg2+ as a cofactor, releasing abundant molecular beacons containing ASOs. ASOs hybridize with Egr-1 mRNAs, inducing the recovery of Cy5 fluorescence for Egr-1 mRNA imaging and silencing. This nanoantenna achieves a limit of detection of 7.46 fM for in vitro assay. It can profile Egr-1 mRNA level in diverse human cells, and distinguish Egr-1 mRNA level in breast cancer tissues and healthy counterparts. Moreover, it can be applied for high-contrast imaging of Egr-1 mRNA in vivo, efficient gene silencing, and enhanced tumor ablation, with promising applications in smart gene therapeutics and precise nanomedicines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biosensors and Bioelectronics
工程技术-电化学
CiteScore
20.80
自引率
7.10%
发文量
1006
审稿时长
29 days
期刊介绍:
Biosensors & Bioelectronics, along with its open access companion journal Biosensors & Bioelectronics: X, is the leading international publication in the field of biosensors and bioelectronics. It covers research, design, development, and application of biosensors, which are analytical devices incorporating biological materials with physicochemical transducers. These devices, including sensors, DNA chips, electronic noses, and lab-on-a-chip, produce digital signals proportional to specific analytes. Examples include immunosensors and enzyme-based biosensors, applied in various fields such as medicine, environmental monitoring, and food industry. The journal also focuses on molecular and supramolecular structures for enhancing device performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


