在实验性仓鼠攻击模型中对一种新型和两种许可的犬用利什曼原虫疫苗的功效进行评估
IF 2.5
3区 医学
Q2 PARASITOLOGY
引用次数: 0
摘要
引入和目的内脏利什曼病是影响人类和犬类的最严重的利什曼病。疫苗接种是最具成本效益的疾病控制方法。我们的目的是将一种实验性候选疫苗和两种上市疫苗(CaniLeish®和LetiFend®)与使用仓鼠模型的对照组进行比较。方法以两种寄生虫抗原成分为基础制备候选疫苗配方。第一种是从多诺瓦利什曼原虫中分离的纯化部分,称为病灶甘露糖配体(FML),第二种是从多诺瓦利什曼原虫培养物中提取的排泄/分泌蛋白(E/S)。该蛋白在一种由三种不同佐剂(CPG, Quil a和胆固醇/乙醇溶液)组成的新型佐剂体系中配制。主要疗效变量为寄生虫血症和皮肤病变的临床观察。次要疗效变量为体重、寄生虫载量和靶组织病理变化。结果与对照组相比,新型候选疫苗的有效性优于其他两种疫苗,其基础是较低的寄生虫血症、靶组织中的寄生虫载量、组织病理学样本中较少的无梭菌存在、较少的皮肤病变和较少的体重减轻。在血液(寄生虫血症)和靶组织(肝脏、皮肤和脾脏)中的寄生虫负荷情况下,这些差异具有统计学意义。结论与对照组相比,新型候选疫苗在仓鼠模型中的疗效优于CaniLeish®或Letifend®。这些结果证实,该候选疫苗可能比目前市售的犬利什曼病疫苗更有效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
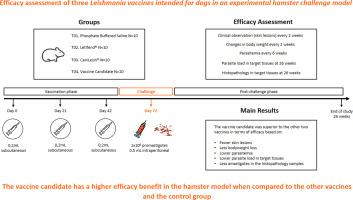
Efficacy assessment of a novel and two licensed Leishmania vaccines intended for dogs in an experimental hamster challenge model
Introduction and aim
Visceral leishmaniasis is the most severe form of leishmaniasis affecting humans and canine species. Vaccination is the most cost-effective approach to disease control. Our aim is to compare an experimental vaccine candidate and two marketed vaccines (CaniLeish® and LetiFend®) with a control group using a hamster model.
Methods
The vaccine candidate formulation was based on two parasite antigenic components. The first is a purified fraction isolated from Leishmania donovani named fucose mannose ligand (FML) and the second is excretory/secretory protein (E/S) derived from a L. donovani culture. The proteins are formulated in a novel adjuvant system composed of three different adjuvants (CPG, Quil A and Cholesterol/Ethanol solution). The primary efficacy variables were parasitemia and clinical observations of skin lesions. The secondary efficacy variables were changes in body weight, parasite load and histopathology in target tissues.
Results
Compared to the control group, the novel vaccine candidate was superior to the other two vaccines in terms of efficacy based on lower parasitemia, parasite load in target tissues, less presence of amastigotes in the histopathology samples, fewer skin lesions and less bodyweight loss. These differences were statistically significant in the case of parasite load in blood (parasitemia), and target tissues (liver, skin, and spleen).
Conclusion
The novel vaccine candidate may have a higher efficacy benefit in the hamster model than CaniLeish® or Letifend® when compared to the control group. These results confirm the vaccine candidate as a potentially more efficacious alternative to the current commercially available vaccines against canine leishmaniasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta tropica
医学-寄生虫学
CiteScore
5.40
自引率
11.10%
发文量
383
审稿时长
37 days
期刊介绍:
Acta Tropica, is an international journal on infectious diseases that covers public health sciences and biomedical research with particular emphasis on topics relevant to human and animal health in the tropics and the subtropics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


