细胞外囊泡:抗病毒功能及其在治疗和疫苗中的应用
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
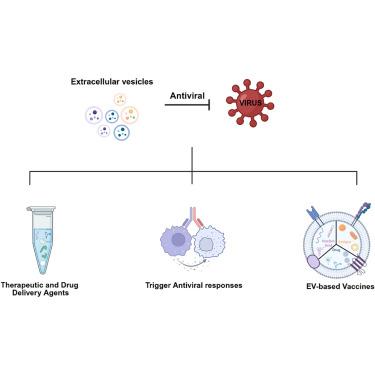
细胞外囊泡(EVs)是由几乎所有细胞类型分泌的纳米级膜状囊泡,在病毒感染过程中表现出双重调节机制:它们通过包装整个感染性病毒颗粒或功能病毒基因组促进感染传播,同时传递免疫活性物质参与宿主抗病毒免疫反应。此外,ev本身可以发挥直接的抗病毒作用,例如通过与病毒粒子竞争结合宿主细胞受体,如磷脂酰丝氨酸(PS)受体。作为天然纳米载体,电动汽车因其优越的生物相容性、低免疫原性和有效穿透生理屏障而成为新型的治疗递送载体。通过表面修饰和货物装载等工程策略,电动汽车能够靶向递送治疗剂,包括抗病毒分子和基因编辑工具,有效抑制病毒复制并增强宿主免疫反应。此外,它们独特的抗原呈递机制和免疫刺激特性强调了疫苗开发的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Extracellular vesicles: Antiviral functions and applications in therapeutics and vaccines
Extracellular vesicles (EVs) are nanoscale membranous vesicles secreted by nearly all cell types, exhibiting dual regulatory mechanisms during viral infection: they facilitate infection spread by packaging whole infectious viral particles or functional viral genomes while concurrently delivering immunoactive substances to participate in host antiviral immune responses. Additionally, EVs themselves can exert direct antiviral effects, such as by competing with virions for binding to host cell receptors such as phosphatidylserine (PS) receptor. As natural nanocarriers, EVs have emerged as novel therapeutic delivery vehicles owing to their superior biocompatibility, low immunogenicity, and efficient penetration across physiological barriers. Through engineering strategies such as surface modification and cargo loading, EVs enable targeted delivery of therapeutic agents, including antiviral molecules and gene-editing tools, effectively suppressing viral replication and enhancing host immune responses. Furthermore, their unique antigen-presenting mechanisms and immunostimulatory properties underscore significant potential in vaccine development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


