噬菌体-哺乳动物细胞相互作用的转录组学分析揭示了不同的噬菌体免疫条形码
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
噬菌体-哺乳动物细胞相互作用组是揭示噬菌体可能对人类产生的各种影响的新兴研究前沿。在这里,我们研究了六种不同的鲍曼不动杆菌噬菌体与A549上皮细胞和RAW264.7巨噬细胞的串扰。我们的研究结果表明,噬菌体内化率取决于噬菌体类型和细胞系。值得注意的是,足病毒噬菌体的内化率最高,而肌病毒噬菌体的内化率最低。内化噬菌体对细胞内细菌保持显著活性。RNA测序显示,噬菌体处理的A549细胞显示抗炎转录特征(免疫条形码)。相反,用虹膜病毒噬菌体CK02或足病毒噬菌体CK21处理的巨噬细胞显示出抗炎特征,而用肌病毒噬菌体CK12处理的巨噬细胞保持促炎反应。此外,两种噬菌体处理的细胞都表现出细胞繁殖和增殖途径的下调,这表明噬菌体与哺乳动物细胞相互作用的维度尚未得到充分探索。总之,我们的发现突出了噬菌体对哺乳动物细胞作用的复杂性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
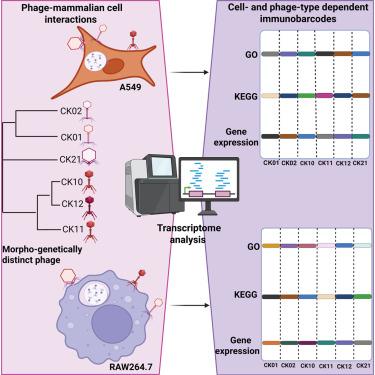
Transcriptomic analysis of phage-mammalian cell interaction reveals diverse phage immunobarcodes
The phage-mammalian cell interactome is an emerging research frontier essential for uncovering the diverse impacts phages may have on humans. Here, we investigate the crosstalk of six distinct Acinetobacter baumannii phages with A549 epithelial cells and RAW264.7 macrophages. Our findings indicate that phage internalization rate varies depending on the phage type and the cell line. Notably, podovirus phage demonstrates the highest rate of internalization, while myovirus phage exhibits the lowest. Internalized phages maintain significant activity against intracellular bacteria. RNA sequencing revealed that phage-treated A549 cells display anti-inflammatory transcriptional signatures (immunobarcodes). Conversely, macrophages treated with siphovirus phage CK02 or podovirus phage CK21 demonstrate an anti-inflammatory profile, while those treated with myovirus phage CK12 maintain a pro-inflammatory response. Moreover, both phage-treated cells exhibit the downregulation of cellular reproduction and proliferation pathways, pointing to underexplored dimensions of phage-mammalian cell interactions. Altogether, our findings highlight the complexity of phage effects on mammalian cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


