FTO抑制通过下调铁下垂激活因子ACSL4和促纤维化因子TGFBI来减轻肾纤维化
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
肾纤维化(RF)是慢性肾脏疾病(CKD)的主要病理特征和潜在的治疗靶点,慢性肾脏疾病是一种普遍存在的健康问题,给卫生保健系统带来了高昂的经济负担。与假手术对照组相比,在小鼠单侧输尿管梗阻(UUO)后,脂肪量和肥胖相关(FTO)抑制,无论是遗传还是药理学上,都显著减少胶原沉积、脂质过氧化和铁下垂标志物的表达。在小鼠和人肾上皮细胞以及人胚胎干细胞衍生的肾类器官中,FTO抑制通过下调铁下垂驱动因子ACSL4来减少脂质过氧化和活性氧的产生,从而减少了erastin诱导的铁下垂。此外,FTO抑制直接下调TGFBI,这与UUO后M2巨噬细胞积累减少密切相关。我们的研究结果为靶向FTO减轻梗阻相关性肾损伤患者的RF提供了强有力的理论依据,从而降低CKD的患病率和相关的治疗成本,提高生活质量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
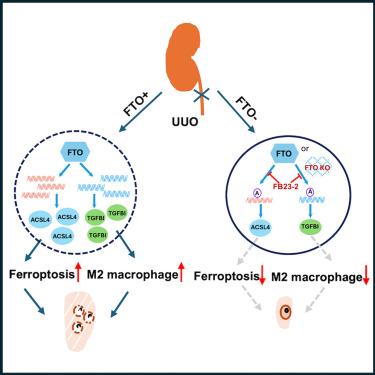
FTO inhibition attenuates renal fibrosis by downregulating ferroptosis activator ACSL4 and profibrotic factor TGFBI
Renal fibrosis (RF) is the main pathological feature and a potential therapeutic target of chronic kidney disease (CKD), a prevalent health problem causing a high economic burden to the health care system. Fat mass and obesity-associated (FTO) inhibition, either genetically or pharmacologically, significantly reduced collagen deposition, lipid peroxidation, and ferroptosis marker expression after unilateral ureteral obstruction (UUO) compared with sham-operated controls in mice. In murine and human kidney epithelial cells as well as in human embryonic stem cell-derived kidney organoids, FTO inhibition reduced erastin-induced ferroptosis by decreasing lipid peroxidation and reactive oxygen species production by downregulating the ferroptosis driver ACSL4. Moreover, FTO inhibition directly downregulated TGFBI, which was strongly associated with reduced M2 macrophage accumulation after UUO. Our results provide a strong rationale for targeting FTO to alleviate RF in patients subjected to obstruction-related kidney injury, thereby reducing the prevalence of CKD and associated treatment costs and improving the quality of life.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


