复方高子板片通过肠脑轴缓解小鼠焦虑和抑郁样行为:参与雌激素信号通路
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景/目的复方高子板片(FGZBP)是一种维吾尔族中成药,具有改善抑郁症状的作用。然而,其对焦虑抑郁共发病的影响及其潜在机制的探讨尚不够深入。本研究探讨了FGZBP对应激模型小鼠焦虑和抑郁样行为的影响,以及其潜在机制。研究设计/方法在本研究中,用不同剂量的FGZBP治疗慢性不可预测的轻度应激小鼠。透射电镜观察海马突触超微结构,染色观察脑和肠组织的生理变化。同时,通过脑和结肠组织的RNA测序,评估FGZBP对脑和结肠转录的调节作用,并探讨其可能的机制。采用RT-qPCR和ELISA分析血清和脑组织中的炎症标志物和候选代谢物,采用16s rRNA序列检测肠道微生物组的变化。结果大剂量FGZBP能有效缓解大鼠抑郁和焦虑行为。它改善了CUMS小鼠的脑组织和肠道损伤,上调了与雌激素受体通路相关的基因表达。从而逆转小胶质细胞的激活,减少促炎介质的分泌,提高BDNF和PSD-95的表达,这是高剂量FGZBP所实现的。同时,高剂量FGZBP可调节肠道微生态紊乱,减轻CUMS引起的结肠氧化应激和生理变化。综上所述,FGZBP通过调节肠-脑轴雌激素信号通路,逆转CUMS小鼠的炎症和氧化应激,从而减少抑郁和焦虑行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
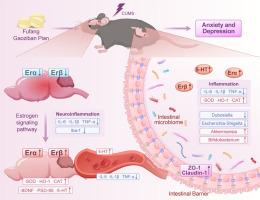
Fufang Gaoziban Pian alleviates anxiety and depression-like behavior in mice via the gut-brain axis: Involvement of the estrogen signaling pathway
Background/Objective
Fufang Gaoziban Pian (FGZBP) is a Uyghur patent Chinese medicine, and it has been shown to improve depressive symptoms. However, its impact on co-morbidity of anxiety and depression, as well as its exploration of the potential mechanisms, have not been probed in sufficient depth. This research explored the impacts of FGZBP on anxiety and depression-like behaviors, along with its underlying mechanisms in mice exposed to a stress model.
Research design/Methodology
In this study, mice with chronic unpredictable mild stress were treated with different doses of FGZBP. The ultrastructure of hippocampal synapses was assessed via transmission electron microscopy, and physiological changes in brain and intestinal tissues were evaluated using staining. Simultaneously, the modulatory effects of the FGZBP on brain and colonic transcriptions were assessed by RNA sequencing of brain and colon tissues, and potential mechanisms were explored. Additionally, inflammatory markers and candidate metabolites in serum and brain tissue were analyzed using RT-qPCR and ELISA, using 16 s rRNA sequence to test changes of intestinal microbiome.
Results
The results demonstrated that the high-dose FGZBP effectively alleviated depressive and anxious behaviors. It ameliorated brain histological and intestinal damage, upregulated the expression of genes linked with the estrogen receptor pathway in CUMS mice. Thereby reversing microglial activation, diminishing the secretion of pro-inflammatory mediators, and raising the expressions of BDNF and PSD-95, which was achieved by the high-dose FGZBP. Simultaneously, the high-dose FGZBP dysregulated the intestinal microecological disturbances and alleviated colonic oxidative stress and physiological changes induced by CUMS.
Conclusion
In summary, our findings suggested that the FGZBP reversed inflammation and oxidative stress in CUMS mice by regulating estrogen signaling pathways via the gut-brain axis, thereby reducing depressive and anxious behaviors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


