自消毒黏附壳聚糖纳米喷雾治疗按需抗生素预防卫生保健相关感染
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
医疗保健相关感染(HAIs)是一个重要的临床挑战,它显著增加了患者的发病率和医疗保健成本。虽然ph响应性抗菌纳米颗粒(NPs)已成为有前景的治疗平台,但目前的策略受到生理条件下组织粘附性差和药物过早释放的阻碍。在这里,我们提出了基于儿茶酚偶联壳聚糖和动态Cu2+配位的可喷雾黏附抗菌NPs,用于预防感染引发的万古霉素释放。这种多功能治疗系统独特地整合了强大的组织粘附性,感染反应性药物释放,优越的生物相容性和有效的抗菌活性,不像传统方法那样单独处理各个方面。电喷涂制备的儿茶酚- cu2 +配位壳聚糖NPs (Cat-CS@Van NPs)通过多种相互作用机制表现出优异的粘接性能。关键是,万古霉素在酸性pH值(5.0)下的释放量比生理pH值(7.4)高2.7倍,通过酸化诱导儿茶酚- cu2 +络合物的不稳定,在8小时内释放量达到75.5%。Cat-CS@Van NPs还保留了优异的喷雾性,没有结构降解,并且在洗涤后保持了很强的组织粘附性。体外和离体实验显示,该材料对革兰氏阳性和革兰氏阴性细菌均具有广谱抗菌活性,能够在最低抑菌负荷浓度下完全根除细菌,并有效防止细菌在医疗设备界面上的易位。此外,与对照组相比,体内伤口感染模型显示出更好的治疗效果,增强了伤口愈合。因此,我们的可喷雾Cat-CS@Van NP系统为现场定向抗菌预防提供了一种有希望的方法,以实现即时和可持续的杀菌作用,有效预防HAI。本文章由计算机程序翻译,如有差异,请以英文原文为准。
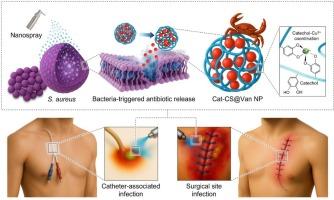
Self-disinfecting adhesive chitosan nanospray therapeutics for on-demand antibiotic prophylaxis against healthcare-associated infections
Healthcare-associated infections (HAIs) present a critical clinical challenge that significantly increases patient morbidity and healthcare costs. While pH-responsive antibacterial nanoparticles (NPs) have emerged as promising therapeutic platforms, current strategies are hampered by poor tissue adhesion and premature drug release under physiological conditions. Here, we propose sprayable adhesive antibacterial NPs based on catechol-conjugated chitosan with dynamic Cu2+ coordination for infection-triggered vancomycin release in HAI prevention. This multifunctional therapeutic system uniquely integrates robust tissue adhesion, infection-responsive drug release, superior biocompatibility, and potent antibacterial activity unlike conventional approaches that address individual aspects separately. The catechol-Cu2+ coordinated chitosan NPs (Cat-CS@Van NPs) fabricated via electrospraying exhibited superior mucoadhesive properties through multiple interaction mechanisms. Crucially, vancomycin release at acidic pH (5.0) was ∼2.7-fold higher than at physiological pH (7.4), achieving ∼75.5 % release within 8 h through acidification-induced destabilization of catechol-Cu2+ complexes. The Cat-CS@Van NPs also retained excellent sprayability without structural degradation and maintained strong tissue adhesion after washing. In vitro and ex vivo assays showed broad-spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria, enabling complete bacterial eradication at the minimum inhibitory loading concentration and effective prevention of bacterial translocation across medical device interfaces. Furthermore, in vivo wound infection models demonstrated improved therapeutic outcomes with enhanced wound healing compared to controls. Thus, our sprayable Cat-CS@Van NP system provides a promising approach for site-directed antibacterial prophylaxis to achieve instant and sustainable bactericidal action for effective HAI prevention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


